10-K: Annual report [Section 13 and 15(d), not S-K Item 405]
Published on September 22, 2025
Table of Contents
Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
Transition Report under Section 13 or 15(d) of the Securities Exchange Act of 1934 |
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) | |
(Address of principal executive offices) |
(Zip Code) | |
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered | ||
| Large Accelerated Filer | ☐ | Accelerated Filer | ☐ | |||
Non-Accelerated Filer |
☒ | Smaller Reporting Company | ||||
| Emerging Growth Company | ||||||
Table of Contents
RESTATMENT
EXPLANATORY NOTE
In connection with the preparation of Benitec Biopharma Inc.’s (the “Company”) Annual Report on Form 10-K for the fiscal year ended June 30, 2025, the Company determined that in prior periods it had not appropriately recorded certain non-cash share-based compensation expenses.
As previously announced in the Current Report on Form 8-K filed with the U.S. Securities and Exchange Commission (the “SEC”) on September 12, 2025, the Audit Committee of the Board of Directors of the Company (the “Board”), after consultation with the Company’s management and Baker Tilly US, LLP, the Company’s independent registered public accounting firm, concluded that the following financial statements should no longer be relied upon because of such misstatements related to accounting for share-based compensation expense; (i) the Company’s unaudited consolidated financial statements as of and for the three months and nine months ended March 31, 2025, contained in its Quarterly Report on Form 10-Q for the quarter ended March 31, 2025, originally filed with the SEC on May 14, 2025, and (ii) the Company’s unaudited consolidated financial statements as of and for the three and six months ended December 31, 2024, contained in its Quarterly Report on Form 10-Q for the quarter ended December 31, 2024, originally filed with the SEC on February 14, 2025 (collectively, the “Non-Reliance Periods”).
As a result of these misstatements, the Company is restating financial information for the Non-Reliance Periods. All restated financial information for the Non-Reliance Periods is included in this Annual Report on Form 10-K and the Company has not filed, and does not intend to file, amendments to any of its filings that it has previously filed with the SEC.
Restatement Background
The errors and corrective adjustments identified by the Company are non-cash in nature and resulted from the migration, in November 2023, of equity awards data to a new information recording system used to calculate the Company’s share-based compensation expense, which was incorrectly configured resulting in understatements of share-based compensation expense, which in turn led to understatements of additional paid-in capital, accumulated deficit, net loss and loss per share. The impact was immaterial to the Company’s previously issued financial statements prior to the quarter ended December 31, 2024, but the cumulative impact of the incorrect configuration had a material effect on the unaudited consolidated financial statements as of and for the quarterly periods ended December 31, 2024 and March 31, 2025, the Prior Financial Statements.
Refer to Note 3, Restatement of Prior Period Financial Statements, in the accompanying Consolidated Financial Statements included in Part II, Item 8 of this Form 10-K for additional information, including the impact on the specific accounts.
Internal Control Considerations
In connection with the restatement, management has re-evaluated the effectiveness of the Company’s internal controls over financial reporting. The Audit Committee of the Board, with concurrence of management, has concluded that, in light of the errors described above, a material weakness exists in the Company’s internal control over financial reporting as of June 30, 2025. Management is actively taking steps to remediate the material weakness in the Company’s internal control over financial reporting. For a discussion of management’s consideration of the Company’s disclosure controls and procedures, internal control over financial reporting, and the material weakness identified, see Part II, Item 9A Controls and Procedures of this Form 10-K.
Items Restated in this Form 10-K
The following items have been restated, as appropriate, to correct the errors noted above:
| • | Part II, Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations |
| • | Part II, Item 8, Financial Statements and Supplementary Data |
| • | Part IV, Item 15, Exhibits and Financial Statement Schedules |
Table of Contents
BENITEC BIOPHARMA INC.
ANNUAL REPORT ON FORM 10-K
TABLE OF CONTENTS
Table of Contents
PART I
ABOUT THIS ANNUAL REPORT
Unless the context otherwise requires, the terms “Benitec,” the “Company,” “we,” “us,” “our” and similar terms used in this Annual Report on Form 10-K refer to Benitec Biopharma Inc., a Delaware corporation, and its subsidiaries (including Benitec Limited). Any references to “Benitec Limited” or “BBL” refer to Benitec Biopharma Limited, an Australian corporation.
All references to “$” in this Annual Report refer to U.S. dollars. Any references to “A$” in this Annual Report mean Australian dollars. As of June 30, 2025, the rate of exchange of U.S. dollars to Australian dollars was 1.5265 AUD.
Our fiscal year-end is June 30. References to a particular “fiscal year” are to our fiscal year ended June 30 of that calendar year.
Except as otherwise stated, all share and earnings per share amounts presented in this Annual Report reflect the impact of the 1-for-17 reverse stock split of the Company’s common stock effective July 26, 2023.
INDUSTRY AND MARKET DATA
This Annual Report includes information with respect to market and industry conditions and market share from third-party sources or based upon estimates using such sources when available. We believe that such information and estimates are reasonable and reliable. We also believe the information extracted from publications of third- party sources has been accurately reproduced. However, we have not independently verified any of the data from third-party sources. Similarly, our internal research is based upon our understanding of industry conditions, and such information has not been verified by any independent sources.
TRADEMARKS AND TRADENAMES
We have proprietary and licensed rights to trademarks used in this Annual Report which are important to our business, many of which are registered under applicable intellectual property laws. Our trademarks include:
| • | BENITEC BIOPHARMA® |
| • | BENITEC® |
| • | GIVING DISEASE THE SILENT TREATMENT® |
| • | SILENCING GENES FOR LIFE® |
Solely for convenience, trademarks and trade names referred to in this Annual Report appear without the “®” or “™” symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent possible under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, any other companies. Each trademark, trade name or service mark of any other company appearing in this Annual Report is the property of its respective holder.
1
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report contains forward-looking statements that are subject to a number of risks and uncertainties, many of which are beyond our control. All statements, other than statements of historical fact included in this Annual Report, regarding our strategy, future operations, financial position, projected costs, prospects, plans and objectives of management are forward-looking statements. When used in this Annual Report, the words “could,” “believe,” “anticipate,” “intend,” “estimate,” “expect,” “may,” “continue,” “predict,” “potential,” “project,” or the negative of these terms, and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain such identifying words. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. These risks, uncertainties and factors include:
| • | the success of our plans to develop and potentially commercialize our product candidates; |
| • | the timing of the completion of preclinical studies and clinical trials; |
| • | the timing and sufficiency of patient enrollment and dosing in any future clinical trials; |
| • | the timing of the availability of data from our clinical trials; |
| • | the timing and outcome of regulatory filings and approvals; |
| • | the development of novel AAV vectors; |
| • | our potential future out-licenses and collaborations; |
| • | the plans of licensees of our technology; |
| • | the clinical utility and potential attributes and benefits of ddRNAi and our product candidates, including the potential duration of treatment effects and the potential for a “one shot” cure; |
| • | our intellectual property position and the duration of our patent portfolio; |
| • | expenses, ongoing losses, future revenue, capital needs and needs for additional financing, and our ability to access additional financing given market conditions and other factors; |
| • | the length of time over which we expect our cash and cash equivalents to be sufficient to execute on our business plan; |
| • | unanticipated delays; |
| • | further research and development and the results of clinical trials possibly being unsuccessful or insufficient to meet applicable regulatory standards or warrant continued development; |
| • | the ability to enroll sufficient numbers of subjects in clinical trials; |
| • | determinations made by the U.S. Food and Drug Administration and other governmental authorities; |
| • | regulatory developments in the United States of America; |
| • | our ability to protect and enforce our patents and other intellectual property rights; |
| • | our dependence on our relationships with our collaboration partners and other third parties; |
| • | the efficacy or safety of our products and the products of our collaboration partners; |
| • | the acceptance of our products and the products of our collaboration partners in the marketplace and market competition; |
| • | sales, marketing, manufacturing and distribution requirements; |
2
Table of Contents
| • | greater than expected expenses, expenses relating to litigation or strategic activities; |
| • | the impact of, and our ability to remediate, the identified material weakness in our internal controls over financial reporting; |
| • | our ability to satisfy our capital needs through increasing revenue and obtaining additional financing; and |
| • | the impact of local, regional and national and international economic conditions and events; |
as well as other risks detailed under the caption “Risk Factors” in this Annual Report and in other reports filed with the SEC. Although we believe that we have a reasonable basis for each forward-looking statement contained in this Annual Report, we caution you that these statements are based on a combination of facts and important factors currently known by us and our expectations of the future, about which we cannot be certain.
We have based the forward-looking statements included in this Annual Report on information available to us on the date of this Annual Report or on the date thereof. Except as required by law we undertake no obligation to revise or update any forward-looking statements, whether as a result of new information, future events or otherwise. You are advised to consult any additional disclosures that we may make directly to you or through reports that we, in the future, may file with the SEC, including annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K.
All forward-looking statements included herein or in documents incorporated herein by reference are expressly qualified in their entirety by the cautionary statements contained or referred to elsewhere in this Annual Report.
| Item 1. | Business. |
Company Overview
We endeavor to become the leader in discovery, development, and commercialization of therapeutic agents capable of addressing significant unmet medical need via the application of the silence and replace approach to the treatment of genetic disorders.
Benitec Biopharma Inc. (“Benitec” or the “Company” or in the first person, “we” or “our”) is a clinical-stage biotechnology company focused on the advancement of novel genetic medicines with headquarters in Hayward, California. The proprietary platform, called DNA-directed RNA interference, or ddRNAi, combines RNA interference, or RNAi, with gene therapy to create medicines that facilitate sustained silencing of disease-causing genes following a single administration. The unique therapeutic constructs also enable the simultaneous delivery of wildtype replacement genes, facilitating the proprietary “silence and replace” approach to the treatment of genetically defined diseases. We are developing a silence and replace-based therapeutic (BB-301) for the treatment of Oculopharyngeal Muscular Dystrophy (OPMD), a chronic, life-threatening genetic disorder.
BB-301 is a silence and replace-based genetic medicine currently under development by Benitec. BB-301 is an AAV-based gene therapy designed to permanently silence the expression of the disease-causing gene (to slow, or halt, the biological mechanisms underlying disease progression in OPMD) and to simultaneously replace the mutant gene with a wildtype gene (to drive restoration of function in diseased cells). This fundamental therapeutic approach to disease management is called “silence and replace.” The silence and replace mechanism offers the potential to restore the normative physiology of diseased cells and tissues and to improve treatment outcomes for patients suffering from the chronic, and potentially fatal, effects of OPMD. BB-301 has been granted Orphan Drug Designation in the United States and the European Union.
The targeted gene silencing effects of RNAi, in conjunction with the durable transgene expression achievable via the use of modified viral vectors, imbues the silence and replace approach with the potential to produce permanent silencing of disease-causing genes along with simultaneous replacement of the wild type gene
3
Table of Contents
function following a single administration of the proprietary genetic medicine. We believe that this novel mechanistic profile of the current and future investigational agents developed by Benitec could facilitate the achievement of robust and durable clinical activity while greatly reducing the frequency of drug administration traditionally expected for medicines employed for the management of chronic diseases. Additionally, the achievement of permanent gene silencing and gene replacement may significantly reduce the risk of patient non-compliance during the course of medical management of potentially fatal clinical disorders. We will require additional financing to progress our product candidates through to key inflection points.
Our proprietary technology platforms are designated as DNA-directed RNA interference, or “ddRNAi”, and “silence and replace.” ddRNAi is designed to produce permanent silencing of disease-causing genes, by combining RNA interference, or RNAi, with viral delivery agents typically associated with the field of gene therapy (i.e., viral vectors). Modified AAV vectors are employed to deliver genetic constructs which encode short hairpin RNAs that are, then, serially expressed and processed to produce siRNA molecules within the transduced cell for the duration of the life of the target cell. These newly introduced siRNA molecules drive permanent silencing of the expression of the disease-causing gene. The silence and replace approach further bolsters the biological benefits of permanent silencing of disease-causing genes by incorporating multifunctional genetic constructs within the modified AAV vectors to create an AAV-based gene therapy agent that is designed to silence the expression of disease-causing genes (to slow, or halt, the underlying mechanism of disease progression) and to simultaneously replace the mutant genes with normal, “wildtype” genes (to drive restoration of function in diseased cells). This fundamentally distinct therapeutic approach to disease management offers the potential to restore the underlying physiology of the treated tissues and, in the process, improve treatment outcomes for patients suffering from the chronic and, potentially, fatal effects of diseases like Oculopharyngeal Muscular Dystrophy (OPMD).
Traditional gene therapy is defined by the introduction of an engineered transgene to correct the pathophysiological derangements derived from mutated or malfunctioning genes. Mutated genes can facilitate the intracellular production of disease-causing proteins or hamper the production of critical, life-sustaining, proteins. The introduction of a new transgene can facilitate the restoration of production of normal proteins within the diseased cell, thus restoring natural biological function. Critically, the implementation of this traditional method of gene therapy cannot eliminate the expression, or the potential deleterious effects of, the underlying mutant gene (as mutant proteins may be continually expressed and aggregate or drive the aggregation of other native proteins within the diseased cell). In this regard, the dual capabilities of the proprietary silence and replace approach to silence a disease-causing gene via ddRNAi and simultaneously replace the wild type activity of a mutant gene via the delivery of an engineered transgene could facilitate the development of differentially efficacious treatments for a range of genetic disorders.
Overview of RNAi and the siRNA Approach
The mutation of a single gene can cause a chronic disease via the resulting intracellular production of a disease-causing protein (i.e., an abnormal form of the protein of interest), and many chronic and/or fatal disorders are known to result from the inappropriate expression of a single gene or multiple genes. In some cases, genetic disorders of this type can be treated exclusively by “silencing” the intracellular production of the disease- causing protein through well-validated biological approaches like RNA interference (“RNAi”). RNAi employs small nucleic acid molecules to activate an intracellular enzyme complex, and this biological pathway temporarily reduces the production of the disease-causing protein. In the absence of the disease-causing protein, normal cellular function is restored and the chronic disease that initially resulted from the presence of the mutant protein is partially or completely resolved. RNAi is potentially applicable to over 20,000 human genes and a large number of disease- causing microorganism-specific genes.
4
Table of Contents
Figure 1
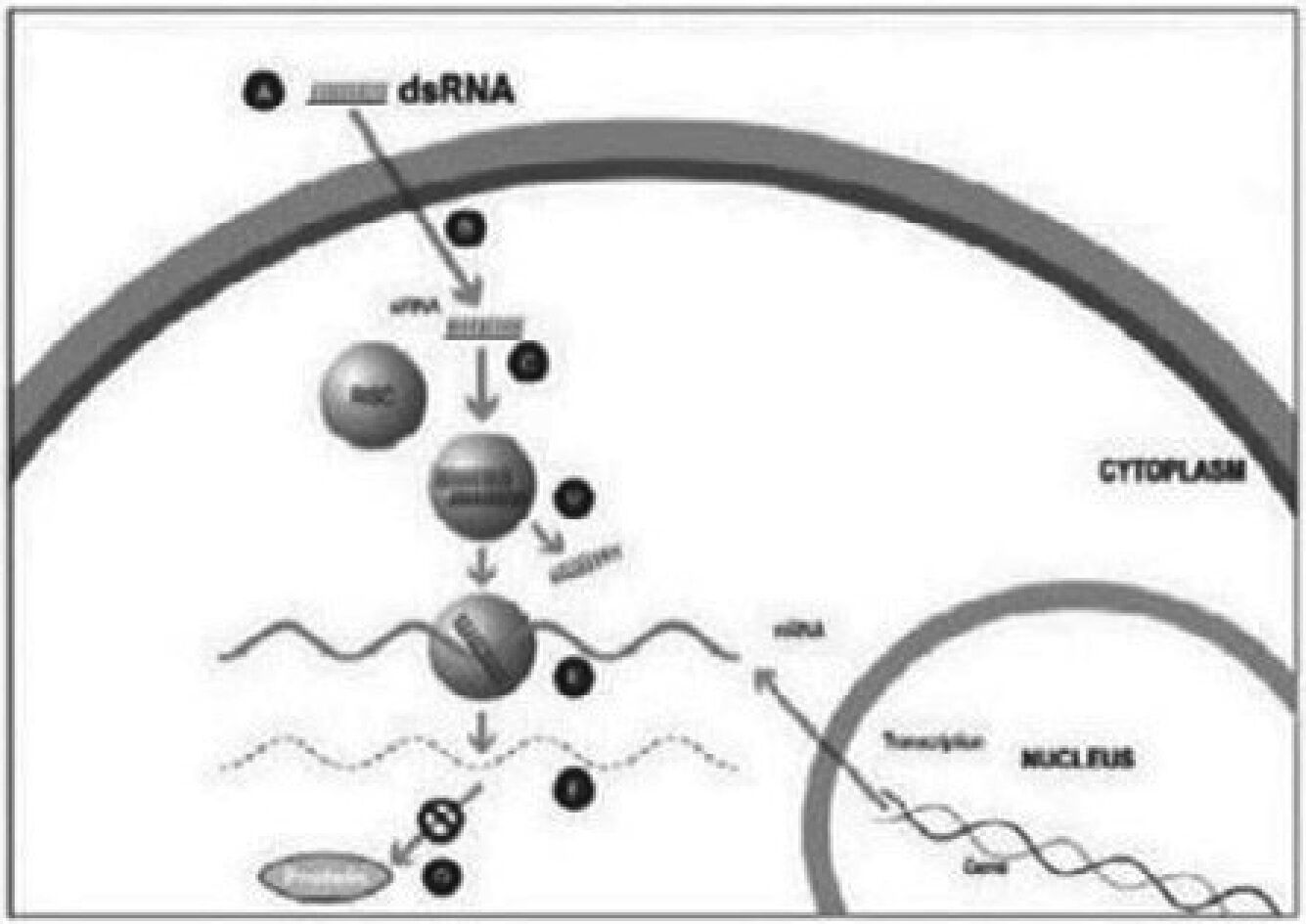
A small double stranded RNA, or dsRNA, molecule (A, Figure 1), comprising one strand known as the sense strand and another strand known as the antisense strand, which are complementary to each other, is synthesized in the laboratory. These small dsRNAs are called small interfering RNAs, or siRNAs. The sequence of the sense strand corresponds to a short region of the target gene mRNA. The siRNA is delivered to the target cell (B, Figure 1), where a group of enzymes, referred to as the RNA-Induced Silencing Complex, or RISC, process the siRNA (C, Figure 1), where one of the strands (usually the sense strand) is released (D, Figure 1). RISC uses the antisense strand to find the mRNA that has a complementary sequence (E, Figure 1) leading to the cleavage of the target mRNA (F, Figure 1). As a consequence, the output of the mRNA (protein production) does not occur (G, Figure 1). Several companies, including Alnylam Pharmaceuticals Inc. (“Alnylam”), utilize this approach in their RNAi product candidates.
Importantly, many genetic disorders are not amenable to the traditional gene silencing approach outlined in Figure 1, as the diseased cells may produce a mixture of the wild type protein of interest and the disease-causing mutant variant of the protein, and the underlying genetic mutation may be too small to allow for selective targeting of the disease-causing variant of the protein through the use of siRNA-based approaches exclusively. In these cases, it is extraordinarily difficult to selectively silence the disease-causing protein without simultaneously silencing the wild type intracellular protein of interest whose presence is vital to the conduct of normal cellular functions.
Our proprietary silence and replace technology utilizes the unique specificity and robust gene silencing capabilities of RNAi while overcoming many of the key limitations of siRNA-based approaches to disease management.
In the standard RNAi approach, double-stranded siRNA is produced synthetically and, subsequently, introduced into the target cell via chemical modification of the RNA or alternative methods of delivery. While efficacy has been demonstrated in several clinical indications through the use of this approach, siRNA-based approaches maintain a number of limitations, including:
| • | Clinical management requires repeat administration of the siRNA-based therapeutic agent for multiple cycles to maintain efficacy; |
5
Table of Contents
| • | Long-term patient compliance challenges due to dosing frequencies and treatment durations; |
| • | Therapeutic concentrations of siRNA are not stably maintained because the levels of synthetic siRNA in the target cells decrease over time; |
| • | Novel chemical modifications or novel delivery materials are typically required to introduce the siRNA into the target cells, making it complicated to develop a broad range of therapeutics agents; |
| • | Potential adverse immune responses, resulting in serious adverse effects; |
| • | Requirement for specialized delivery formulations for genetic disorders caused by mutations of multiple genes; and |
| • | siRNA acts only to silence genes and cannot be used to replace defective genes with normally functioning genes. |
Our Approach to the Treatment of Genetic Diseases—ddRNAi and Silence and Replace
Our proprietary silence and replace approach to the treatment of genetic diseases combines RNAi with wild type gene replacement to drive permanent silencing of disease-causing genes and concomitant restoration of functional wild type genes following a single administration of the therapeutic agent. Benitec employs ddRNAi in combination with classical gene therapy (i.e., transgene delivery via viral vectors) to overcome several of the fundamental limitations of RNAi.
The silence and replace approach to the treatment of genetic disorders employs adeno-associated viral vectors (“AAVs”) to deliver genetic constructs which may, after a single administration to the target tissues:
| • | Chronically express RNAi molecules inside of the target, diseased, cells (to serially silence the intracellular production of mutant, disease-causing, protein and the wild type protein of interest); |
| • | Simultaneously drive the expression of a wild type variant of the protein of interest (to restore native intracellular biological processes); and |
| • | AAV vectors can accommodate the multi-functional DNA expression cassettes containing the engineered wild type transgenes and the novel genes encoding short hairpinRNA/microRNA molecules (shRNA/miRNA) that are required to support the development of therapeutic agents capable of the achievement of the goals of the silence and replace approach to therapy. |
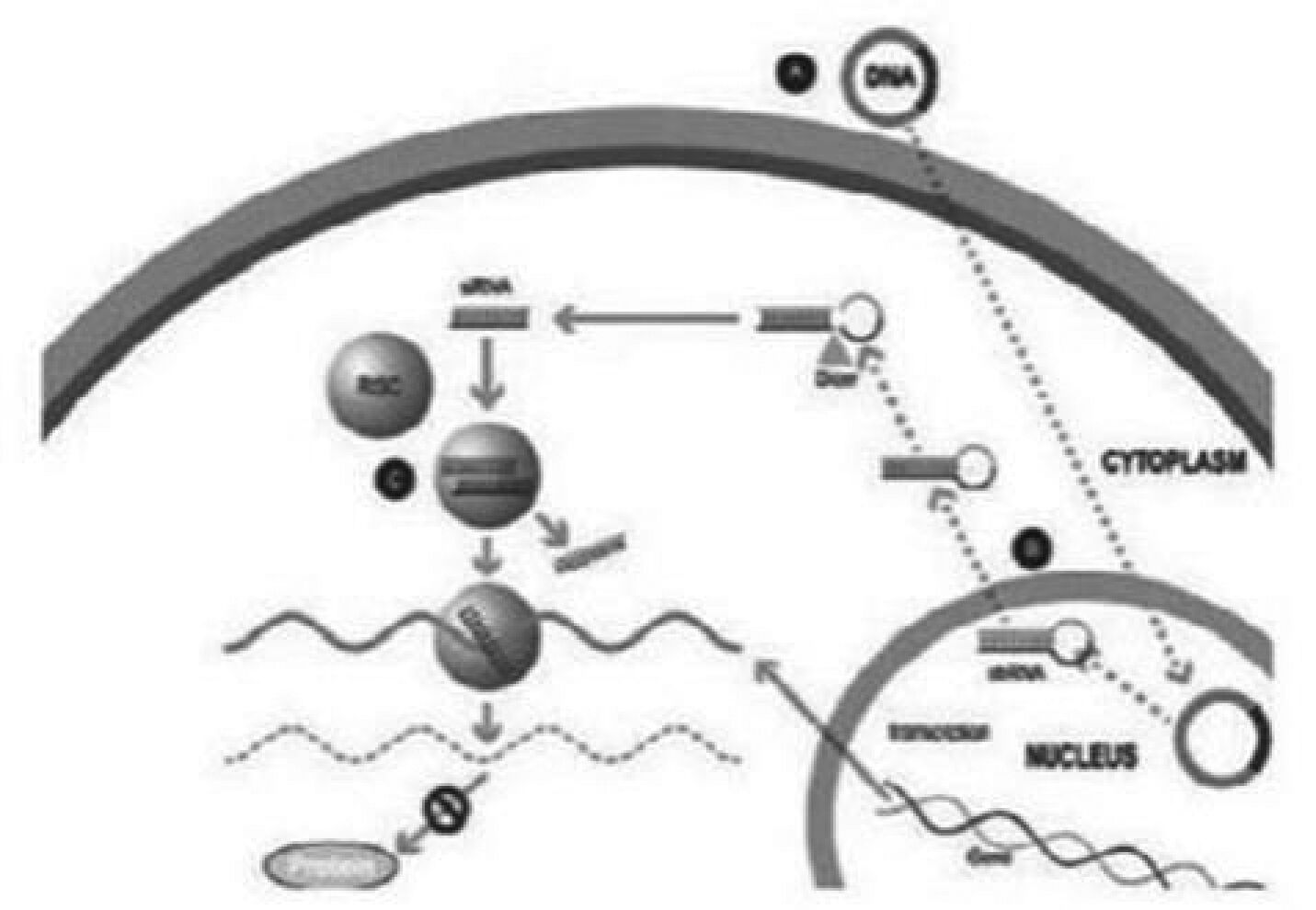
Our silence and replace technology utilizes proprietary DNA expression cassettes to foster continuous production of gene silencing shRNAs and wild type proteins (via expression of the wild type transgene). A range of viral and non-viral gene therapy vectors can be used to deliver the DNA construct into the nucleus of the target cell and, upon delivery, shRNA molecules are expressed and subsequently processed by intracellular enzymes into siRNA molecules that silence the expression of the mutant, disease-causing protein (Figure 2).
In the silence and replace approach (Figure 2):
| • | A DNA construct is delivered to the nucleus of the target cell by a gene therapy vector (A) such as an AAV vector; |
| • | Once inside of the nucleus, the DNA construct drives the continuous production of shRNA molecules (B) which are processed by an enzyme called Dicer into siRNAs (C); |
| • | The processed siRNA is incorporated into RISC and silences the target gene using the same mechanism shown in Figure 1; and |
| • | When the DNA expression cassette is additionally comprised of a wild type transgene, upon entry of the DNA construct into the nucleus of the target cell via the use of the AAV vector, the DNA construct also drives the continuous production of wild type protein (to restore native intracellular biological processes). |
6
Table of Contents
Figure 2
Our strategy is to discover, develop and commercialize treatments that leverage the capabilities of ddRNAi and the silence and replace approach to disease management.
For selected product candidates, at the appropriate stage, we may collaborate with large biopharmaceutical companies to further co-develop and, if approved, commercialize our ddRNAi-based and silence and replace-based products to achieve broad clinical and commercial distribution. For specific clinical indications that we deem to be outside of our immediate areas of focus, we will continue to out-license, where appropriate, applications of our ddRNAi and silence and replace technology to facilitate the development of differentiated therapeutics, which could provide further validation of our proprietary technology and approach to disease management.
Our cash and cash equivalents will be deployed for the advancement of our product candidate BB-301 for the treatment of OPMD-derived dysphagia, including the natural history lead-in study and the Phase 1b/2a BB-301 treatment study, for the continued advancement of development activities for other existing and new product candidates, for general corporate purposes and for strategic growth opportunities.
Oculopharyngeal Muscular Dystrophy—OPMD
OPMD is an insidious, autosomal-dominant, late-onset degenerative muscle disorder that typically presents in patients at 40-to-50 years of age. The disease is characterized by progressive swallowing difficulties (dysphagia) and eyelid drooping (ptosis). OPMD is caused by a specific mutation in the poly(A)-binding protein nuclear 1, or PABPN1, gene. OPMD is a rare disease; however, patients have been diagnosed with OPMD in at least 33 countries. Patient populations suffering from OPMD are well-identified, and significant geographical clustering has been noted for patients with this disorder, which could simplify clinical development and global commercialization efforts.
BB-301 is an AAV-based gene therapy designed to silence the expression of disease-causing genes (to slow, or halt, the underlying mechanism of disease progression) and to simultaneously replace the mutant genes with normal, “wildtype” genes (to drive restoration of function in diseased cells). This fundamental therapeutic
7
Table of Contents
approach to disease management is called “silence and replace” and this biological mechanism offers the potential to restore the underlying physiology of the treated tissues and, in the process, improve treatment outcomes for patients suffering from the chronic and, potentially, fatal effects of Oculopharyngeal Muscular Dystrophy (OPMD). BB-301 has been granted Orphan Drug Designation in the United States and the European Union.
Our Pipeline
The following table sets forth our current product candidate and the development status:
Table 1. Pipeline: Oculopharyngeal Muscular Dystrophy
We are developing BB-301 for the treatment of Oculopharyngeal Muscular Dystrophy (OPMD)-related dysphagia. The Investigational New Drug (IND) application for BB-301 was approved to proceed by the U.S. Food and Drug Administration in June 2023. The first study subject was safely treated in the BB-301 Phase 1b/2a clinical trial (NCT06185673) in November 2023. The second study subject was safely treated in February 2024. The third study subject was safely treated in October 2024. The fourth study subject was safely treated in December 2024. The fifth study subject was safely treated in February 2025, and the sixth study subject was safely treated in April 2025. BB-301 is the lead investigational gene therapy agent under development by Benitec, and the key attributes of BB-301 are outlined in Figure 3.
8
Table of Contents
Figure 3
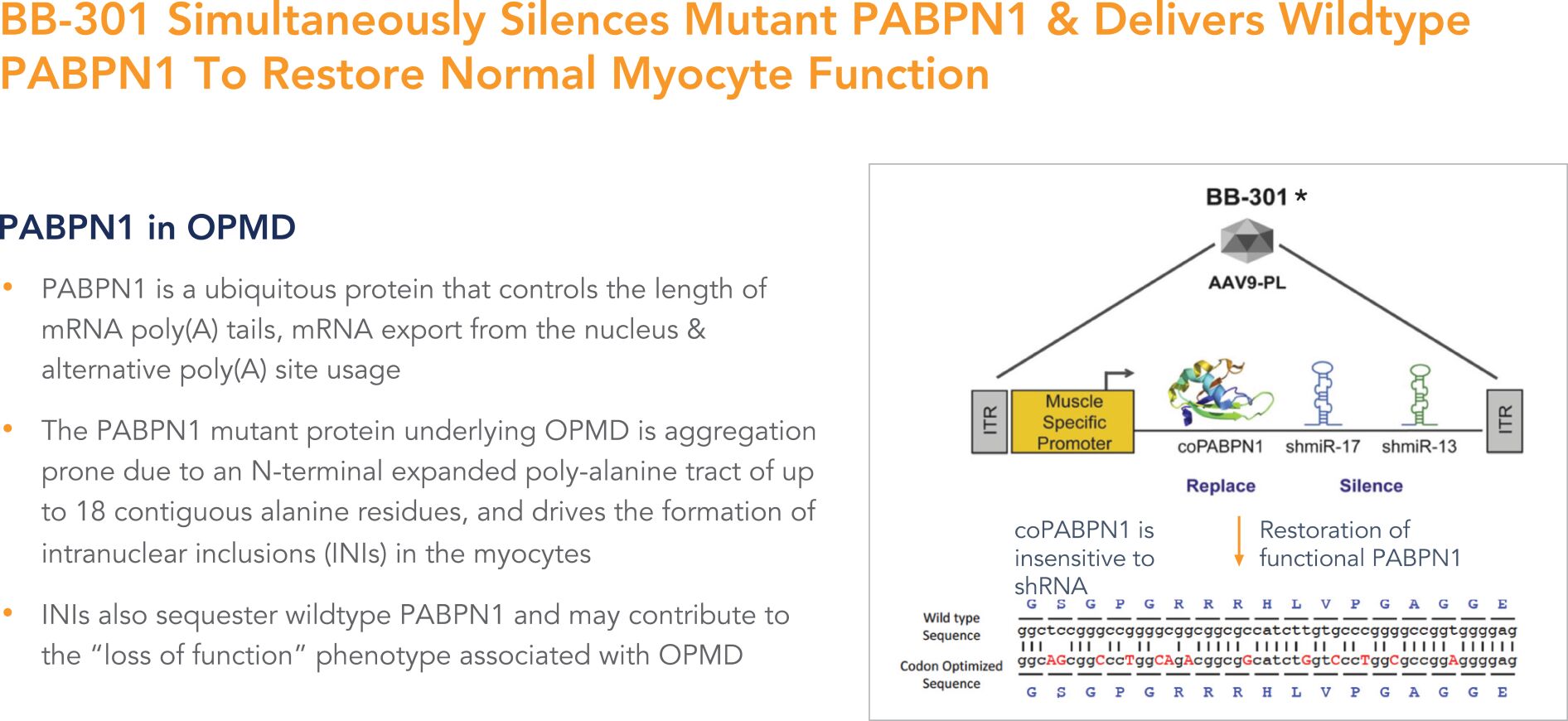
BB-301 is a first-in-class genetic medicine employing the “silence and replace” approach for the treatment of OPMD. OPMD is an insidious, autosomal-dominant, late-onset, degenerative muscle disorder that typically presents in patients at 40-to-50 years of age. The disease is characterized by progressive swallowing difficulties (dysphagia) and eyelid drooping (ptosis). OPMD is caused by a specific mutation in the poly(A)-binding protein nuclear 1 gene (PABPN1).
OPMD is a rare disease, however, patients have been diagnosed with OPMD in at least 33 countries. Patient populations suffering from OPMD are well-identified, and significant geographical clustering has been noted for patients with this disorder. Each of these attributes could facilitate efficient clinical development and global commercialization of BB-301.
PABPN1 is a ubiquitous factor that promotes the interaction between the poly(A) polymerase and CPSF (cleavage and polyadenylation specificity factor) and, thus, controls the length of mRNA poly(A) tails, mRNA export from the nucleus, and alternative poly(A) site usage. The characteristic genetic mutation underlying OPMD results in trinucleotide repeat expansion(s) within exon 1 of PABPN1 and results in an expanded poly-alanine tract at the N-terminal end of PABPN1. The mutation generates a protein with an N-terminal expanded poly-alanine tract of up to 18 contiguous alanine residues, and the mutant protein is prone to the formation of intranuclear aggregates designated as intranuclear inclusions (INIs). The INIs that sequester wildtype PABPN1 may contribute to the “loss of function” phenotype associated with OPMD.
No therapeutic agents are approved for the treatment of OPMD. Additionally, there are no surgical interventions available to OPMD patients that modify the natural history of the disease, which is principally comprised of chronic deterioration of swallowing function. BB-301 has received Orphan Drug Designation in the United States and the European Union and, upon achievement of regulatory approval for BB-301 in these respective jurisdictions, the Orphan Drug Designations would provide commercial exclusivity independent of intellectual property protection. While OPMD is a rare medical disorder, we believe the commercial opportunity for a safe and efficacious therapeutic agent in this clinical indication exceeds $1 billion over the course of the commercial life of the product.
BB-301 is our Lead, Silence and Replace-Based, OPMD Therapeutic Agent
BB-301 is composed of a modified AAV serotype 9 (AAV9) capsid that expresses a bifunctional construct under the control of a single muscle specific Spc5-12 promoter to achieve co-expression of both the codon-optimized
9
Table of Contents
PABPN1 mRNA and two shmiR molecules directed against wild type and mutant PABPN1. BB-301 is designed to correct the genetic defect underlying OPMD following a single localized administration.
BB-301—Design and Mechanism of Action
BB-301 is designed to target two distinct regions of the PABPN1 mRNA to accomplish gene silencing via the concomitant expression of two distinct shmiRs from a single DNA construct (Figure 4). BB-301 is also engineered to drive the simultaneous expression of a codon-optimized, siRNA-resistant, version of the wild type PABPN1 gene (Figure 4).
Figure 4
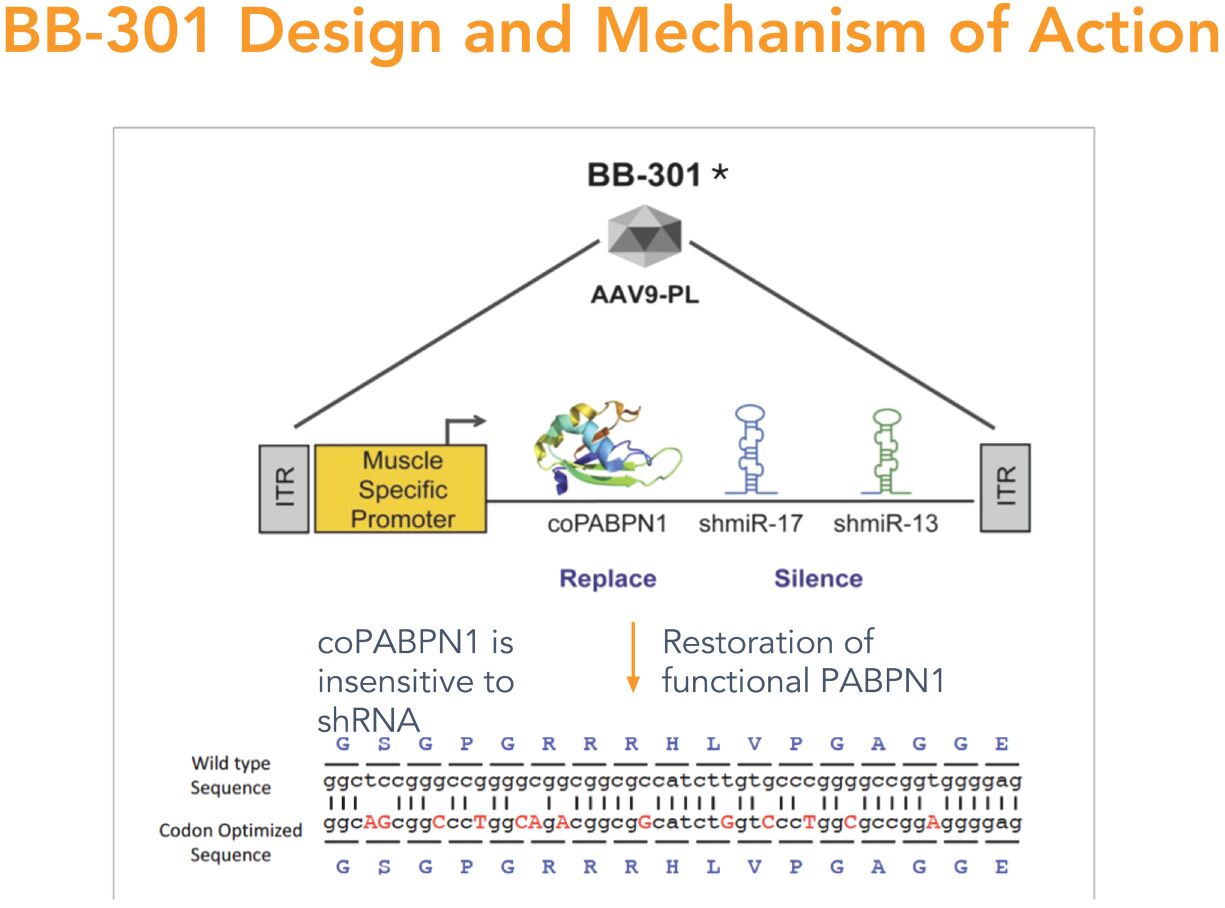
Summary of the Key Regulatory Interactions:
| • | In June 2023 the U.S. Food and Drug Administration (FDA) cleared the Investigational New Drug (IND) application for BB-301 which allowed dosing of BB-301 to begin for OPMD subjects that are eligible for enrollment into the Phase 1b/2a treatment study (NCT06185673) described below. |
Operational Updates
The key milestones related to the development of BB-301 for the treatment of OPMD, along with other corporate updates, are outlined below:
BB-301 Clinical Development Program Overview:
| • | The BB-301 clinical development program will be conducted in the United States, and the primary elements of the program are summarized below: |
| • | The program will comprise approximately 76 weeks of follow-up which we anticipate will consist of: |
| • | The OPMD Natural History (NH) Study: 6-month pre-treatment observation periods for the evaluation of baseline disposition and natural history of OPMD-derived dysphagia (swallowing impairment) in each study participant. |
| • | Dosing with BB-301: 1-day of BB-301 dosing to initiate participation in the Phase 1b/2a single-arm, open-label, sequential, dose-escalation cohort study (NCT06185673). BB-301 will be delivered directly to the pharyngeal muscles of each study subject. |
10
Table of Contents
| • | Phase 1b/2a Treatment Evaluation: 52-weeks of post-dosing follow-up for conclusive evaluation of the primary and secondary endpoints of the BB-301 Phase 1b/2a treatment study (NCT06185673), with interim safety and efficacy results expected to be available at the end of each 180-day period following the administration of BB-301. |
| • | The OPMD NH Study will characterize the level of dysphagia borne by each OPMD subject at baseline and assess subsequent progression of dysphagia via the use of the following quantitative radiographic measures (i.e., videofluoroscopic swallowing studies or “VFSS”). The VFSS outlined below collectively provide objective assessments of global swallowing function and the function of the pharyngeal constrictor muscles (i.e., the muscles whose functional deterioration drives disease progression in OPMD): |
| • | Total Pharyngeal Residue %(C2-4)2 |
| • | Pharyngeal Area at Maximum Constriction (PhAMPC) |
| • | Dynamic Imaging Grade of Swallowing Toxicity Scale (DIGEST) |
| • | Vallecular Residue %(C2-4)2, Pyriform Sinus Residue %(C2-4)2, and Other Pharyngeal Residue %(C2-4)2 |
| • | Normalized Residue Ratio Scale (NRRSv, NRRSp) |
| • | Pharyngeal Construction Ratio (PCR) |
| • | The NH study will also employ clinical measures of global swallowing capacity and oropharyngeal dysphagia, along with two distinct patient-reported outcome instruments targeting the assessment of oropharyngeal dysphagia. |
| • | Upon the achievement of 6-months of follow-up in the NH Study, participants will, potentially, be eligible for enrollment into the BB-301 Phase 1b/2a treatment study (NCT06185673). |
| • | BB-301 Phase 1b/2a Treatment Study (NCT06185673): |
| • | This first-in-human (FIH) study will evaluate the safety and clinical activity of intramuscular doses of BB-301 administered to subjects with OPMD. |
| • | The primary endpoint of the FIH study will be safety. |
| • | Secondary endpoints are designed to determine the impact of BB-301 on swallowing efficiency, swallowing safety, and pharyngeal constrictor muscle function in subjects diagnosed with OPMD with dysphagia via the use of serial clinical and videofluoroscopic assessments. Critically, each of the clinical and videofluoroscopic assessments employed in the FIH study will be equivalent to those employed for the NH study to facilitate comparative clinical and statistical analyses for each study subject. |
| • | The primary and secondary endpoints will be evaluated during each 90-day period following BB-301 intramuscular injection (Day 1). |
| • | The NH of dysphagia observed for each OPMD NH Study participant, as characterized by the VFSS and clinical swallowing assessments carried out during the NH Study, will serve as the baseline for comparative assessments of safety and efficacy of BB-301 upon rollover from the NH Study onto the BB-301 Phase 1b/2a Treatment Study (NCT06185673). |
Intellectual Property
Benitec seeks to actively procure rights to and protect the intellectual property and proprietary technology that it believes is important to its business. Such intellectual property rights include, but are not limited to, patents claiming our proprietary ddRNAi and silence and replace technologies, and specific product candidates employing those technologies, as well as know-how and trade secrets related to our product candidates and proprietary technology.
11
Table of Contents
ddRNAi-based treatment for OPMD
Benitec’s patent portfolio for OPMD includes five active patent families relating to shRNA and shmiRs targeting PABPN1 (the causative gene for OPMD), ‘silence and replace’ therapeutics and treatment strategies for OPMD, as well as interoperative delivery methods and delivery devices for use in such treatment strategies . These five families cover: (i) the individual shmiRs comprised within the OPMD therapeutic candidate, BB-301, under development at Benitec, (ii) the ‘silence and replace’ construct within BB-301, (iii) treatment strategies for OPMD that silence PABPN1 which is causative for OPMD and replace with functional PABPN1, (iv) Benitec’s proprietary AAV vector for delivery of BB-301, (v) Benitec’s proprietary injection needle for delivery of BB-301 to the pharyngeal muscle of OPMD patients, (vi) pre-filled multi-injection devices used for delivery of BB-301, and (vii) an interoperative method to enable delivery of BB-301 to the pharyngeal muscle of OPMD patients. In this regard, BB-301 is a ‘silence and replace’ construct encoding two shmiRs targeting the endogenous PABPN1 (including variants causative of OPMD) internally designated shmiR-13 and shmiR-17, as well as a codon-optimized PABPN1 replacement construct, the transcript of which is not targeted by shmiR-13 and shmiR-17. Both shmiRs and the codon-optimized PABPN1 replacement construct are under the control of a muscle-specific promoter and packaged within an AAV9 vector with a modified capsid protein. BB-301 is administered to the pharyngeal muscle via an interoperative method using a proprietary injection needle design for optimized delivery. Multi-injection delivery devices fitted with the proprietary injection needle and pre-filled with appropriate dosage volumes of BB-301 have been developed for delivery of BB-301 to the pharyngeal muscle of affected subject via the intraoperative method.
The first patent family, entitled “Reagents for treatment of oculopharyngeal muscular dystrophy (OPMD) and use thereof (OPMD family #1)”, arose out of a collaboration with Royal Holloway University of London (RHUL) and relates to three shRNA target regions within PABPN1. RHUL assigned its ownership interests in this patent family to Benitec, and the PCT application and the related U.S. priority document were filed solely in the name of Benitec. This patent family is directed to RNAi agents targeting specific regions within mutant PABPN1 variants causative of OPMD, as well as use of those RNAi agents in combination with PABPN1 replacement constructs to treat OPMD. More specifically, this family includes claims covering shmiR17 of BB-301 This patent family entered the national/regional phase in October/November 2018.
The second patent family, entitled “Reagents for treatment of oculopharyngeal muscular dystrophy (OPMD) and use thereof (OPMD family #2)” relates to a second set of target regions within PABPN1, as well as the ‘silence and replace’ construct BB-301 under development at Benitec. The PCT application and the related U.S. priority document were filed solely in the name of Benitec, and this family entered the national/regional phase in June/July 2019. This patent family is directed to RNAi agents targeting specific regions within mutant PABPN1 variants causative of OPMD, as well as ‘silence and replace’ constructs and use of same for treatment of OPMD. More specifically, this family includes claims covering shmiR13 and shmiR17 of BB-301 separately and in combination, as well as the full BB-301 ‘silence and replacement’ construct.
A third patent family, entitled “Methods for Treating Oculopharyngeal Muscular Dystrophy (OPMD) (OPMD family #3)” was filed by Benitec’s former licensee, Axovant Therapeutics, on Benitec’s behalf to pursue claims which are broadly directed to the ‘silence and replace’ treatment concept for OPMD, relying on RNAi agents to knockdown PABPN1 and replacement with functional PABPN1 which is not targeted by the RNAi agents. The claims in this application are not limited to the regions targeted by BB-301. The PCT application and the related U.S. priority document were filed solely in the name of Benitec, and this family entered the national/regional phase in April/May 2021. Whilst a number of patent applications remain pending, OPMD family #3 has been passively abandoned in line with Benitec’s evolving IP strategy for the OPMD program. As such, OPMD family #3 is no longer considered an active patent family for the OPMD program.
A fourth patent family, entitled “Methods for Treating Oculopharyngeal Muscular Dystrophy (OPMD) (OPMD family #4)” has been filed to specifically claim the OPMD therapeutic candidate developed by Benitec, BB-301, encompassing the ‘silence and replace’ construct (described herein) packaged with Benitec’s proprietary AAV9 vector having a modified phospholipase (PLA2) domain within its capsid (See following section). This PCT
12
Table of Contents
application and the related U.S. priority document were filed solely in the name of Benitec, and this family entered the national/regional phase in August/September 2022.
A fifth patent family, entitled “Device and methods for administering a therapeutic composition to the pharyngeal muscle (OPMD family #5)” has been filed to capture (i) the interoperative delivery method developed by Benitec for administration of BB-301 to the pharyngeal muscle, (ii) the proprietary needle developed by Benitec for use in the interoperative delivery method, (iii) multi-injection devices fitted with Benitec’s proprietary needle and pre-filled with BB-301, and (iv) sets of the multi-injection devices suitable for treatment of a single patient. This patent family includes supporting preliminary clinical data generated for the first two patients participating in the Phase 2a clinical trial (the results of which are described herein). This patent family proceed as a PCT application, and both the PCT application and the related U.S. priority document were filed solely in the name of Benitec.
AAV with modified phospholipase domain
The Benitec patent portfolio includes a single patent family, entitled “Adeno-associated virus (AAV) with modified phospholipase domain,” which relates to an AAV having a modified phospholipase (PLA2) domain in the capsid. The modified AAV will be used as the delivery system for the OPMD therapeutic, BB-301. The PCT application and the related U.S. priority document were filed solely in the name of Benitec, and this family entered the national/regional phase in February/March 2021.
We are aware of a third party patent directed to AAV vectors that expires in 2026. In the event we receive regulatory marketing approval before the expiration date it may be necessary for us to obtain a license to the patent in order to commercialize. We cannot guarantee the availability of the license or that it can be obtained on commercially reasonable terms.
Know-How
In addition to patent protection of ddRNAi and other technology and our product candidates, we also rely on proprietary know-how that is not patentable or that we elect not to patent, as valuable intellectual property for our business. This know-how is related to the areas of, among others, identifying nucleic acid targets for ddRNAi technology and designing ddRNAi constructs for targeting preferred genes. We have implemented a number of security measures designed to safeguard our know-how including limiting access to our research facilities, databases and networks. We also seek to protect our know-how by way of confidentiality agreements when engaging with external providers for progressing our pipeline of therapeutic candidates.
Laws and Regulations Regarding Patent Terms
The term of individual patents depends upon the legal terms of the patents in the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the earliest date of filing a non-provisional (or ‘complete’) patent application. In the United States, a patent term may be shortened if a patent is terminally disclaimed over another patent owned by the same assignee. A patent’s term may also be lengthened by a patent term adjustment (PTA), which compensates a patentee for administrative delays by the USPTO in granting a patent. However, calculation of PTA also takes into account delays by the patentee during patent prosecution, which may partially or completely offset any additional term accorded to account for delays by the USPTO. The patent term of a European patent is 20 years from its complete filing date, which, unlike in the United States, is not subject to patent term adjustments due to delays by the European Patent Office (EPO) or patentee during prosecution.
The term of a patent that covers an FDA-approved drug substance may also be eligible for patent term extension (PTE) as compensation for the portion of the patent term that the patentee is able to commercially exploit the patent due to the lengthy FDA regulatory review process which is required for marketing of the drug substance. The Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Act, permits a PTE
13
Table of Contents
of up to five years beyond the expiration of the patent. The length of the PTE issued is related to the length of time the drug substance is under clinical testing and regulatory review during the term of the patent. However, PTE cannot extend the term of a patent beyond a total of 14 years from the date of marketing approval for the drug substance and only one patent applicable to an approved drug substance may be extended under PTE. Similar provisions are available in Europe and other jurisdictions to extend the term of a patent that covers an approved drug substance, although the eligibility requirements and criteria for calculating the duration of such extensions, vary. In the future, if and when our products receive FDA approval, and/or approval from an equivalent regulatory body in another jurisdiction in which patent protection is sought or obtained, we expect to apply for patent term extensions on patents covering those products.
Trademarks
Our trademarks include registrations for company branding and product names for our pipeline in development. The trademarks that we use in connection with our business include the following:
| Country or Territory |
Trademark (program) |
Application or Registration number |
Status | |||
| USA | BENITEC BIOPHARMA | 86190065 | Registered | |||
| USA | SILENCING GENES FOR LIFE | 86488147 | Registered | |||
| Australia | SILENCING GENES FOR LIFE BENITEC | 1448041 | Registered | |||
| Australia | BIOPHARMA | 1448046 | Registered | |||
| Australia | BENITEC—logo | 1448052 | Registered | |||
| Australia | Nervarna | 1526478 | Registered | |||
| Australia | TRIBETARNA | 1526479 | Registered | |||
| Australia | HEPBARNA | 1526483 | Registered | |||
| International Bureau (WIPO) – designating EU; UK and US | GIVING DISEASE THE SILENT TREATMENT | 1389399 | Registered | |||
| USA | BENITEC | 86795296 | Registered | |||
| USA | GIVING DISEASE THE SILENT TREATMENT | 79226988 | Registered | |||
| European Union | BENITEC | 14680003 | Registered | |||
| Australia | BENITEC | 1728797 | Registered | |||
| Australia | BENITEC | 1103049 | Registered | |||
| Australia | BENITEC | 1103300 | Registered | |||
| Australia | GIVING DISEASE THE SILENT TREATMENT |
1851660 | Registered | |||
| United Kingdom | BENITEC | 3238275 | Registered |
Manufacturing
The manufacture of the biological products required for gene therapy is complex and difficult. We do not currently own or operate manufacturing facilities for the production of preclinical, clinical or commercial quantities of any of our product candidates. We are exploring long-term manufacturing alliances with a number of potential partners to investigate manufacturing processes in order to produce materials at reasonable scale and cost of goods to support future commercialization efforts. We do not have a long-term agreement with any third-party manufacturer, but we plan to establish such a relationship with an appropriate manufacturer to serve our long-term needs.
Manufacturing is subject to extensive regulations that impose various procedural and documentation requirements, which govern record keeping, manufacturing processes and controls, personnel, quality control and quality assurance, among others. Our contract manufacturing organizations manufacture our product candidates under cGMP conditions. cGMP is a regulatory standard for the production of pharmaceuticals that will be used in humans.
14
Table of Contents
Sales and Marketing
We have not yet established sales, marketing or product distribution operations because our product candidates are in preclinical or clinical development. If we receive marketing and commercialization approval for any of our product candidates, we intend to market the product through strategic alliances and distribution agreements with third parties. In certain cases, we may market an approved product directly worldwide or in selected geographical segments. The ultimate implementation of our strategy for realizing the financial value of our product candidates is dependent on the results of clinical trials for our product candidates, the availability of funds and the ability to negotiate acceptable commercial terms with third parties.
Competition
The biopharmaceutical industry is characterized by intense and dynamic competition to develop new technologies and proprietary therapies.
Any product candidates that we successfully develop and commercialize will have to compete with existing therapies and new therapies that may become available in the future. While we believe that our proprietary technology and scientific expertise in gene silencing using ddRNAi provide us with competitive advantages, we face potential competition from many different sources, including larger and better-funded pharmaceutical, specialty pharmaceutical and biotechnology companies, as well as from academic institutions and governmental agencies and public and private research institutions that may develop potentially competitive products or technologies. We are aware of several companies focused on developing gene therapy or gene silencing product candidates.
We are not aware of any companies developing a gene therapy or gene silencing approach for OPMD. Our product candidates, if approved, would also compete with treatments that have already been approved and accepted by the medical community, patients and third-party payers.
Many of our competitors and potential competitors, alone or with their strategic partners, have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development of product candidates, obtaining FDA and other regulatory approvals of treatments and the commercialization of those treatments. Mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated among a smaller number of our competitors. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical study sites and subject registration for clinical studies, as well as in acquiring technologies complementary to, or necessary for, our programs. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
We anticipate that we will face intense and increasing competition as new products enter the market and advanced technologies become available. We expect any treatments that we develop and commercialize to compete on the basis of, among other things, efficacy, safety, convenience of administration and delivery, price, the level of competition and the availability of reimbursement from government and other third party-payers.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. In addition, we expect that our therapeutic products, if approved, will be priced at a significant premium over competitive products and our ability to compete may be affected in many cases by insurers or other third-party payers seeking to encourage the use of competitive products including biosimilar or generic products.
15
Table of Contents
This increasingly competitive landscape may compromise the development of our product candidates.
Government Regulation
This increasingly competitive landscape may compromise the development of our product candidates. As a pharmaceutical and biological product company that wishes to conduct clinical trials and ultimately obtain marketing approval in the United States, we are subject to extensive regulation by the FDA, and other federal, state, and local regulatory agencies. The Federal Food, Drug, and Cosmetic Act, or the FDC Act, the Public Health Service Act, or PHS Act, and their implementing regulations set forth, among other things, requirements for the research, testing, development, manufacture, quality control, safety, effectiveness, approval, labeling, storage, record keeping, reporting, distribution, import, export, advertising and promotion of our products. A failure to comply explicitly with any requirements during the product development, approval, or post-approval periods, may lead to administrative or judicial sanctions. These sanctions could include the imposition by the FDA or an IRB, of a suspension on clinical trials, refusal to approve pending marketing applications or supplements, withdrawal of approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution.
Although the discussion below focuses on regulation in the United States, we anticipate seeking approval for the testing and marketing of our products in other countries. Generally, our activities in other countries will be subject to regulation that is similar in nature and scope as that imposed in the United States, although there can be important differences. Additionally, some significant aspects of regulation in the European Union are addressed in a centralized way through the EMA, but country-specific regulation remains essential in many respects.
Government regulation may delay or prevent testing or marketing of our products and impose costly procedures upon our activities. The testing and marketing approval process, and the subsequent compliance with appropriate statutes and regulations, requires substantial time, effort, and financial resources, and we cannot be certain that the FDA or any other regulatory agency will grant marketing approvals for our products or any future products on a timely basis, if at all. The FDA’s or any other regulatory agency’s policies may change and additional governmental regulations may be enacted that could prevent or delay regulatory approval of our products or any future products or approval of new indications or label changes. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative, judicial, or administrative action, either in the United States or abroad.
Recent Developments in Regulation of Gene Therapy
Government Regulation in the United States
The FDA has provided guidance for the development of gene therapy products. For example, the FDA has established the Office of Tissues and Advanced Therapies (formerly Office of Cellular, Tissue and Gene Therapies) within the Center for Biologics Evaluation and Research (CBER), to consolidate the review of gene therapy and related products, and the Cellular, Tissue and Gene Therapies Advisory Committee to advise CBER on its reviews. In addition, the FDA has issued a growing body of clinical guidelines, chemical, manufacturing and control, or CMC, guidelines, regenerative medicine guidelines and other guidelines, all of which are intended to facilitate industry’s development of gene therapy products.
In 2016, Section 3033 of the 21st Century Cures Act created a new product category called “regenerative medicine advanced therapy”, or the RMAT designation. The RMAT designation gives the sponsor of a new investigational biologic access to increased meeting opportunities with the FDA, in a manner comparable to those offered to sponsors of therapies designated as “breakthrough therapies” by the FDA. Because the designated products meet the criteria for unmet medical need in the treatment of a serious condition, they may be eligible for priority review, in which the initial assessment of the BLA is reduced from 12 months to eight
16
Table of Contents
months, and accelerated approval, which bases approval on an effect on a predictive surrogate endpoint or an intermediate clinical endpoint. RMATs qualifying for such accelerated approval may be able to satisfy licensing requirements through commitment to post-approval clinical studies as well as real-world data such as patient registries and health record analysis. The eligibility of the RMAT-designated product for these expedited programs can be discussed with the FDA at specific development meetings, but we do not know whether any of our current or future product candidates will be eligible for RMAT designation. We believe the increased access to the FDA during early development is a benefit for sponsors, because the typical Type B development meetings are normally restricted to one each at the stages of pre-IND, end of Phase II/pre-Phase III and pre-BLA submission. In addition, the option to qualify for a fast-track program, also based on the potential to serve an unmet medical need in the treatment of a serious condition, allows for a so-called “rolling review” of parts of the BLA, which can be submitted for assessment following agreement of a review timetable with CBER.
The FDA plans to include certain gene therapy products that permanently alter tissue and produce a sustained therapeutic benefit as part of the products that will meet the definition of being eligible to come under the pathway enabled by RMAT designation. RMAT designation enables gene therapy products to access the FDA’s existing expedited programs to help foster the development and approval of gene therapy products. Our product candidates may not be eligible for RMAT designation now or in the future.
In May 2016, the EMA approved a second gene therapy product called Strimvelis, the first approved ex vivo stem cell gene therapy, to treat patients with a very rare disease called ADA-SCID (Severe Combined Immunodeficiency due to Adenosine Deaminase deficiency).
In August 2017, the FDA approved the first gene therapy product in the United States. The FDA approved Kymriah (tisagenlecleucel) for the treatment of certain pediatric and young adult patients with a form of acute lymphoblastic leukemia (ALL). Kymriah is a genetically-modified autologous T-cell immunotherapy. Because of the risk of cytokine release syndrome and neurological events, Kymriah is being approved with a REMS. In December 2017, the FDA approved Luxturna (voretigene neparvovec-rzyl), a gene therapy to treat children and adult patients with an inherited form of vision loss that may result in blindness. Luxturna is the first directly administered gene therapy approved in the United States that targets a disease caused by mutations in a specific gene.
Marketing Approval
In the United States, for premarket approval purposes, the FDA regulates gene therapy products as biologics under the FDC Act, the PHS Act and related regulations.
The steps required before a new biologic may be marketed in the United States generally include:
| • | nonclinical pharmacology and toxicology laboratory and animal tests according to good laboratory practices, or GLPs, and applicable requirements for the humane use of laboratory animals or other applicable regulations; |
| • | submission of an IND application which must become effective before human clinical trials may begin; |
| • | adequate and well-controlled human clinical trials according to GCPs and any additional requirements for the protection of human research subjects and their health information to establish the safety and efficacy of the investigational product for each targeted indication; |
| • | submission of a biologics license application, or BLA, to the FDA; |
| • | FDA’s pre-approval inspection of manufacturing facilities to assess compliance with cGMPs and, if applicable, the FDA’s good tissue practices, or GTPs, for the use of human cellular and tissue products to prevent the introduction, transmission, or spread of communicable diseases; |
17
Table of Contents
| • | FDA’s audit of clinical trial sites that generated data in support of the BLA; and |
| • | FDA approval of a BLA, which must occur before a product can be marketed or sold. |
Product Development Process
Before testing any biologic in humans, the product enters the nonclinical, or preclinical, testing stage. Nonclinical tests include laboratory evaluations of product chemistry, toxicity, and formulation, as well as animal studies to assess the potential safety and activity of the product. The conduct of nonclinical tests must comply with federal regulations and requirements including GLPs.
Where a gene therapy trial is conducted at, or sponsored by, institutions receiving NIH funding for recombinant DNA research, prior to the submission of an IND to the FDA, a protocol and related documentation is submitted to and the trial is registered with the NIH Office of Science Policy, or OSP.
The product sponsor then submits the results of the nonclinical testing, together with manufacturing information, analytical data, any available clinical data or literature, and a proposed clinical protocol, to the FDA in an IND, which is a request for authorization from the FDA to administer an investigational product to humans. Some nonclinical testing may continue even after the IND application is submitted. IND authorization is required before interstate shipping and administration of any new product to humans that is not the subject of an approved BLA. The IND automatically becomes effective 30 days after receipt by the FDA unless the FDA, within the 30-day time period, raises concerns or questions about the conduct of the clinical trial and places the clinical trial on a clinical hold. In such case, the IND sponsor must resolve any outstanding concerns with the FDA before the clinical trial may begin. Further, an IRB for each site proposing to conduct the clinical trial must review and approve the plan for any clinical trial before it commences at that site. If the site has an IBC, it may also have to review and approve the proposed clinical trial. Clinical trials involve the administration of the investigational product to patients under the supervision of qualified investigators following GCPs, requirements meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, investigators, and monitors. Clinical trials are conducted under protocols that detail, among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria, the parameters to be used in monitoring safety, including stopping rules that assure a clinical trial will be stopped if certain adverse events should occur, and the efficacy criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of the IND. The informed written consent of each participating subject is required and the form and content of the informed consent must be approved by each IRB.
The clinical investigation of an investigational product is generally divided into three phases. Although the phases are usually conducted sequentially, they may overlap or be combined in some cases. The three phases of an investigation are as follows:
| • | Phase I includes the initial introduction of an investigational product into humans. Phase I clinical trials may be conducted in patients with the target disease or condition or on healthy volunteers. These studies are designed to evaluate the safety, metabolism, pharmacokinetics and pharmacologic actions of the investigational product in humans, the side effects associated with increasing doses, and if possible, to gain early evidence on effectiveness. During Phase I clinical trials, sufficient information about the investigational product’s pharmacokinetics and pharmacological effects may be obtained to permit the design of Phase II clinical trials. The total number of participants included in Phase I clinical trials varies, but is generally in the range of 20 to 80. |
| • | Phase II includes the controlled clinical trials conducted to evaluate the effectiveness of the investigational product for a particular indication(s) in patients with the disease or condition under study, to determine dosage tolerance and optimal dosage, and to identify possible adverse side effects and safety risks associated with the product. Phase II clinical trials are typically well- controlled, closely monitored, and conducted in a limited patient population, usually involving no more than several hundred participants. Phase IIa trials provide information on the impact of dose ranging on |
| safety, biomarkers and proof of concept, while Phase IIb trials are patient dose-ranging efficacy trials. |
18
Table of Contents
| • | Phase III clinical trials are controlled clinical trials conducted in an expanded patient population at geographically dispersed clinical trial sites. They are performed after preliminary evidence suggesting effectiveness of the investigational product has been obtained, and are intended to further evaluate dosage, clinical effectiveness and safety, to establish the overall benefit-risk relationship of the product, and to provide an adequate basis for product approval. Phase III clinical trials usually involve several hundred to several thousand participants. In most cases, the FDA requires two adequate and well controlled Phase III clinical trials to demonstrate the efficacy of the product. FDA may accept a single Phase III trial with other confirmatory evidence in rare instances where the trial is a large multicenter trial demonstrating internal consistency and a statistically very persuasive finding of a clinically meaningful effect on mortality, irreversible morbidity or prevention of a disease with a potentially serious outcome and confirmation of the result in a second trial would be practically or ethically impossible. |
Annual progress reports detailing the results of the clinical trials must be submitted to the FDA. Written IND safety reports must be promptly submitted to the FDA and the investigators for serious and unexpected adverse events; any findings from other studies, tests in laboratory animals or in vitro testing that suggest a significant risk for human subjects; or any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator brochure. The sponsor must submit an IND safety report within 15 calendar days after the sponsor determines that the information qualifies for reporting. The sponsor also must notify the FDA of any unexpected fatal or life-threatening suspected adverse reaction within seven calendar days after the sponsor’s initial receipt of the information. The FDA typically recommends that sponsors observe subjects for potential gene therapy-related delayed adverse events for a 15-year period, including a minimum of five years of annual examinations followed by 10 years of annual queries, either in person or by questionnaire, of trial subjects.
The decision to terminate a clinical trial of an investigational biologic may be made by the FDA or other regulatory authority, an IRB, an IBC, or institutional ethics committee, or by a company for various reasons. The FDA may place a clinical hold and order the temporary, or permanent, discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial either is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. If the FDA imposes a clinical hold, trials may not recommence without FDA and IRB authorization and then only under terms authorized by the FDA and IRB. In some cases, clinical trials are overseen by an independent group of qualified experts organized by the trial sponsor, or the clinical monitoring board or DSMB. This group provides authorization for whether or not a trial may move forward at designated check points. These decisions are based on the limited access to data from the ongoing trial. The suspension or termination of a clinical trial can occur during any phase of clinical trials if it is determined that the participants or patients are being exposed to an unacceptable health risk. In addition, there are requirements for the registration of ongoing clinical trials of drugs and biologics on public registries and the disclosure of certain information pertaining to the trials as well as clinical trial results after completion.
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, detailed investigational product information is submitted to the FDA in the form of a BLA for a biologic to request marketing approval for the product in specified indications.
Human gene therapy products are a new category of therapeutics. Because this is a relatively new and expanding area of novel therapeutic interventions, there can be no assurance as to the length of the trial period, the number of patients the FDA will require to be enrolled in the trials in order to establish the safety, efficacy, purity and potency of human gene therapy products, or that the data generated in these trials will be acceptable to the FDA to support marketing approval. The NIH and the FDA have a publicly accessible database, the Genetic Modification Clinical Research Information System, which includes information on gene transfer trials and serves as an electronic tool to facilitate the reporting and analysis of adverse events on these trials. Over the last several years the FDA has issued helpful guidance on development of gene therapy products and shown a willingness to work closely with developers, especially with those working in orphan disease areas.
19
Table of Contents
Biologics License Application Approval Process
In order to obtain approval to market a biologic in the United States, a BLA must be submitted to the FDA that provides data from nonclinical studies and clinical trials and manufacturing information establishing to the FDA’s satisfaction the safety, purity, and potency or efficacy of the investigational product for the proposed indication. The BLA must be accompanied by a substantial user fee payment unless a waiver or exemption applies.
The FDA will initially review the BLA for completeness before it accepts it for filing. Under the FDA’s procedures, the agency has 60 days from its receipt of a BLA to determine whether the application will be accepted for filing based on the agency’s threshold determination that the application is sufficiently complete to permit substantive review. After the BLA submission is accepted for filing, the FDA reviews the BLA to determine, among other things, whether the proposed product is safe, pure and potent, which includes determining whether it is effective for its intended use, and whether the product is being manufactured in accordance with cGMP, to assure and preserve the product’s identity, safety, strength, quality, potency and purity, and in accordance with biological product standards. The FDA will inspect the facilities at which the product is manufactured to ensure the manufacturing processes and facilities are in compliance with cGMP requirements and are adequate to assure consistent production of the product within required specifications. For a human cellular or tissue product, the FDA also will not approve the product if the manufacturer is not in compliance with the GTPs. These are FDA regulations that govern the methods used in, and the facilities and controls used for, the manufacture of human cells, tissues, and cellular and tissue based products, which are human cells or tissue intended for implantation, transplant, infusion, or transfer into a human recipient. The primary intent of the GTP requirements is to ensure that cell and tissue based products are manufactured in a manner designed to prevent the introduction, transmission, and spread of communicable disease. Additionally, before approving a BLA, the FDA may inspect one or more clinical sites to assure compliance with GCP.
If the FDA determines the application, manufacturing process or manufacturing facilities are not acceptable, it typically will outline the deficiencies and often will request additional testing or information, or corrective action for a manufacturing facility. This may significantly delay further review of the application. If the FDA finds that a clinical site did not conduct the clinical trial in accordance with GCP, the FDA may determine the data generated by the clinical site should be excluded from the primary efficacy analyses provided in the BLA. Additionally, notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
The FDA may refer applications for novel products or products that present difficult questions of safety or efficacy to an advisory committee. The FDA also may determine a REMS is necessary to assure the safe use of the biologic, in which case the BLA sponsor must submit a proposed REMS. The REMS may include, but is not limited to, a Medication Guide, a communications plan, and other elements to assure safe use, such as restrictions on distribution, prescribing, and dispensing.
After the FDA completes its initial review of a BLA, it will either license, or approve, the product, or issue a complete response letter to communicate that it will not approve the BLA in its current form and to inform the sponsor of changes that the sponsor must make or additional clinical, nonclinical or manufacturing data that must be received before the FDA can approve the application, with no implication regarding the ultimate approvability of the application. If a complete response letter is issued, the sponsor may either resubmit the BLA, addressing all of the deficiencies identified in the letter, or withdraw the application.
The testing and approval process for both a drug and biologic requires substantial time, effort and financial resources and this process may take several years to complete. Data obtained from clinical trials is not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all. We may encounter difficulties or unanticipated costs in our efforts to secure necessary governmental approvals, which could delay or preclude us from marketing our products.
20
Table of Contents
Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biological product candidate intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making a drug or biological product available in the United States for this type of disease or condition will be recovered from sales of the product candidate. Orphan product designation must be requested before submitting a BLA. After the FDA grants orphan product designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan product designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a product candidate that has orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the same drug or biological product for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan exclusivity. Competitors, however, may receive approval of different products for the indication for which the orphan product has exclusivity or obtain approval for the same product but for a different indication than the one for which the orphan product has exclusivity. Orphan product exclusivity also could block the approval of one of our products for seven years if a competitor obtains approval of the same biological product as defined by the FDA or if our product candidate is determined to be contained within the competitor’s product for the same indication or disease. If a drug or biological product designated as an orphan product receives marketing approval for an indication broader than what is designated, it may not be entitled to orphan product exclusivity. Orphan drug status in the European Union has similar, but not identical, benefits.
Expedited Development and Review Programs
The FDA has a fast track program that is intended to expedite or facilitate the process for reviewing new drugs and biological products that meet certain criteria. Specifically, new drugs and biological products are eligible for fast track designation if they are intended to treat a serious or life- threatening condition and demonstrate the potential to address unmet medical needs for the condition. Fast track designation applies to the combination of the product and the specific indication for which it is being studied. The sponsor of a new biologic or drug may request the FDA to designate the biologic or drug as a fast track product at any time during the clinical development of the product. Unique to a fast track product, the FDA may consider for review sections of the marketing application on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the application, the FDA agrees to accept sections of the application and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the application.
Any product submitted to the FDA for marketing, including under a fast track program, may be eligible for other types of FDA programs intended to expedite development and review, such as priority review and accelerated approval. Any product is eligible for priority review if it would, if approved, be a significant improvement in the safety, effectiveness, treatment, diagnosis or prevention of a disease compared to marketed products. The FDA will attempt to direct additional resources to the evaluation of an application for a new biological or drug product designated for priority review in an effort to reduce the review period from 12 to eight months. Additionally, a product may be eligible for accelerated approval. Biological or drug products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may receive accelerated approval, which means that they may be approved on the basis of adequate and well-controlled clinical trials establishing that the product has an effect on a surrogate endpoint that is reasonably likely to predict a clinical benefit, or on the basis of an effect on an intermediate clinical endpoint. As a condition of approval, the FDA may require that a sponsor of a biological or drug product receiving accelerated approval perform adequate and well-controlled post-marketing clinical trials. In addition, the FDA currently requires as a condition for accelerated approval pre-approval of promotional materials, which could
21
Table of Contents
adversely impact the timing of the commercial launch of the product. Lastly, under the provisions of the new Food and Drug Administration Safety and Innovation Act, enacted in 2012, a sponsor can request designation of a product candidate as a “breakthrough therapy.” A breakthrough therapy is defined as a drug or biological that is intended, alone or in combination with one or more other drugs or biological, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the drug or biological may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Drugs and biologicals designated as breakthrough therapies receive the same benefits as drugs and biologicals with Fast Track designation. In addition, the FDA must take certain additional actions, such as intensive guidance on an efficient drug development program (beginning as early as Phase 1), and organizational commitment involving senior managers, intended to expedite the development and review of an application for approval of a breakthrough therapy.
Fast Track designation and breakthrough therapy designation may expedite the product development and approval process, and priority review may expedite the approval process. However, these three paths do not change the standards for approval. Accelerated approval designation changes the standards for product approval and thus may expedite the development and/or approval process.
FDA Additional Requirements
The FDA may require, or companies may pursue, additional clinical trials after a product is approved. These so-called Phase 4 clinical trials may be made a condition to be satisfied for continuing drug and biologic approval. The results of Phase 4 clinical trials can confirm the efficacy of a product candidate and can provide important safety information. In addition, the FDA has expressed statutory authority to require sponsors to conduct post-market studies to specifically address safety issues identified by the agency.
Even if a product candidate receives regulatory approval, the approval may be limited to specific disease states, patient populations and dosages, or might contain significant limitations on use in the form of warnings, precautions or contraindications, or in the form of an onerous REMS, restrictions on distribution, or post-marketing study requirements. Further, even after regulatory approval is obtained, later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. In addition, we cannot predict what adverse governmental regulations may arise from future U.S. or foreign governmental action.
Medical Device Requirements
Our contemplated diagnostics, for use with certain of our therapeutic products, are regulated by FDA as in vitro diagnostic, or IVD, medical devices. Such IVD devices must comply with applicable FDA IVD-specific regulations as well as FDA regulations applicable more broadly to medical devices. These FDA regulations include requirements for registering establishments with FDA; listing IVD devices with FDA; reporting certain adverse events related to IVD devices to FDA; complying with the Quality System Regulation (current good manufacturing practices for devices); labeling IVD devices; and obtaining premarket approval or clearance prior to marketing IVD devices (unless exempt). There are also regulations covering the requirements for investigational devices and the conduct of clinical investigations of devices. Like drugs and biologics, failure to comply with applicable device/IVD requirements can result in legal or administrative enforcement actions against an IVD device firm, its officers or employees, and/or its products.
FDA Post-Approval Requirements
Any products manufactured or distributed by us or on our behalf pursuant to FDA approvals are subject to continuing regulation by the FDA, including requirements for record-keeping, reporting of adverse experiences with the biologic or drug, and submitting biological product deviation reports to notify the FDA of unanticipated
22
Table of Contents
changes in distributed products. Manufacturers are required to register their facilities with the FDA and certain state agencies, and are subject to periodic announced or unannounced inspections by the FDA and certain state agencies for compliance with cGMP requirements, which impose certain quality processes, manufacturing controls and documentation requirements upon us and our third- party manufacturers in order to ensure that the product is safe, has the identity and strength, and meets the quality, purity and potency characteristics that it purports to have. In November 2013, the Drug Quality and Security Act, or DQSA, became law and establishes requirements to facilitate the tracing of prescription drug and biological products through the supply distribution chain. This law includes a number of new requirements that are being implemented over time and require us to devote additional resources to satisfy these requirements, including serializing the product and using new technology and data storage to electronically trace the product from manufacturer to dispenser. If our products are not covered by the serialization and tracing requirements of the DQSA, they may be subject to state pedigree and traceability requirements. We cannot be certain that we or our present or future suppliers will be able to comply with the cGMP and other FDA regulatory requirements. If our present or future suppliers are not able to comply with these requirements, the FDA may halt our clinical trials, refuse to approve any BLA, force us to recall a product from distribution, shut down manufacturing operations or withdraw approval of the applicable BLA. Noncompliance with cGMP or other requirements can result in issuance of warning or untitled letters, civil and criminal penalties, seizures, and injunctive action.
The FDA and other federal and state agencies closely regulate the labeling, marketing and promotion of drugs and biologics. Government regulators, including the Department of Justice and the Office of the Inspector General of the Department of Health and Human Services, as well as state authorities, recently have increased their scrutiny of the promotion and marketing of drugs and biologics. While doctors are free to prescribe any product approved by the FDA for any use, a company can only make claims relating to safety and efficacy of a product that are consistent with FDA approval, and the company is allowed to market a product only for the particular use and treatment approved by the FDA. In addition, any claims we make for our products in advertising or promotion must, among other things, be appropriately balanced with important safety information and otherwise be adequately substantiated. Failure to comply with these requirements can result in adverse publicity, warning or untitled letters, corrective advertising, injunctions, potential civil and criminal penalties, criminal prosecution, and agreements with governmental agencies that materially restrict the manner in which a company promotes or distributes products.
Patent Term Restoration and Marketing Exclusivity
Depending on the timing, duration and specifics of FDA marketing approval of our product candidates, some of our U.S. patents may be eligible for limited patent term extension under the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of a BLA plus the time between the submission date of a BLA and the approval of that application. Only one patent applicable to an approved biological is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent and within sixty days of approval of the biological product. The USPTO, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration.
The Biologics Price Competition and Innovation Act of 2009, which was included within the Patient Protection and Affordable Care Act, created an abbreviated approval pathway for biological products shown to be similar to, or interchangeable with, an FDA-licensed reference biological product, and grants a reference biologic twelve years of exclusivity from the time of first licensure. Biosimilarity, which requires that there be no clinically meaningful differences between the biological product and the reference product in terms of safety, purity, and potency, can be shown through analytical studies, animal studies, and a clinical study or studies. Interchangeability requires that a product is biosimilar to the reference product and the product must demonstrate
23
Table of Contents
that it can be expected to produce the same clinical results as the reference product and, for products administered multiple times, the biologic and the reference biologic may be switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biologic. However, complexities associated with the larger, and often more complex, structure of biological products, as well as the process by which such products are manufactured, pose significant hurdles to implementation that are still being worked out by the FDA.
Pediatric exclusivity is another type of exclusivity in the United States. Pediatric exclusivity, if granted, provides an additional six months of exclusivity to be attached to any existing marketing exclusivity, e.g., twelve year exclusivity, or patent protection for a drug. This six month exclusivity, which runs from the end of other exclusivity protection or patent delay, may be granted based on the voluntary completion of a pediatric trial in accordance with an FDA-issued “Written Request” for such a trial.
Government regulation outside the United States
In addition to regulations in the United States, we will be subject to a variety of regulations in other jurisdictions governing, among other things, clinical trials and any commercial sales and distribution of our products.
Whether or not we obtain FDA approval for a product, we must obtain the requisite approvals from regulatory authorities in foreign countries prior to the commencement of clinical trials or marketing of the product in those countries. Certain countries outside of the United States have a similar process that requires the submission of a clinical trial application much like the IND prior to the commencement of human clinical trials. In the European Union, for example, a request for a clinical trial authorization, or CTA, must be submitted to each country’s national health authority and an independent ethics committee, much like the FDA and the IRB, respectively. Once the CTA is approved in accordance with a country’s requirements, clinical trial development may proceed.
The requirements and process governing the conduct of clinical trials, product approval or licensing, pricing and reimbursement vary from country to country. In all cases, the clinical trials are conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
To obtain regulatory approval of a biological product under European Union regulatory systems, we must submit a marketing authorization application. The application required in the European Union is similar to a BLA in the United States, with the exception of, among other things, country-specific document requirements. The European Union also provides opportunities for market exclusivity. For example, in the European Union, upon receiving marketing authorization, a new biological generally receives eight years of data exclusivity and an additional two years of market exclusivity. If granted, data exclusivity prevents regulatory authorities in the European Union from referencing the innovator’s data to assess a biosimilar application. During the additional two-year period of market exclusivity, a biosimilar marketing authorization can be submitted, and the innovator’s data may be referenced, but no biosimilar product can be marketed until the expiration of the market exclusivity. The innovator may obtain an additional one year of market exclusivity if the innovator obtains an additional authorization during the initial eight year period for one or more new indications that demonstrate significant clinical benefit over existing therapies. This data and market exclusivity regime in the European Union of a total of 10 or 11 years protects against generic competition, but does not protect against the launch of a competing product if the competitor, rather than referencing the clinical data of the originator, has conducted its own clinical trials to support its marketing authorization application.
Orphan drugs in the European Union are eligible for 10-year market exclusivity. This 10-year market exclusivity may be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria for orphan designation, for example, if the product is sufficiently profitable not to justify maintenance of market exclusivity. Additionally, marketing authorization may be granted to a similar product for the same indication at any time if:
| • | the second applicant can establish that its product, although similar, is safer, more effective or otherwise clinically superior; |
24
Table of Contents
| • | the applicant consents to a second orphan medicinal product application; or |
| • | the applicant cannot supply enough orphan medicinal product. |
If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Pharmaceutical Coverage, Pricing and Reimbursement
Sales of our products, when and if approved for marketing, will depend, in part, on the extent to which our products will be covered by third-party payers, such as federal, state, and foreign government healthcare programs, commercial insurance and managed healthcare organizations. These third-party payers are increasingly reducing reimbursements for medical products, biologicals, drugs and services. In addition, the U.S. government, state legislatures and foreign governments have continued implementing cost containment programs, including price controls, restrictions on coverage and reimbursement and requirements for substitution of interchangeable products. Adoption of price controls and cost containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. Decreases in third-party reimbursement for our product candidates or a decision by a third-party payer not to cover our product candidates could reduce physician usage of our products once approved and have a material adverse effect on our sales, results of operations and financial condition.
The containment of healthcare costs has become a priority of federal, state and foreign governments. Third-party payers are increasingly challenging the prices charged for drug products and medical services, examining the medical necessity and reviewing the cost effectiveness of drug products and medical services, in addition to questioning safety and efficacy. If these third-party payers do not consider our products to be cost-effective compared to other available therapies, they may not cover our products after FDA approval or, if they do, the level of payment may not be sufficient to allow us to sell our products at a profit.
Royalties, milestone payments and other license fees
We are required to pay royalties, milestone payments and other license fees in connection with our licensing of intellectual property from third parties, including as discussed below.
Foreign Currency Translation and Other Comprehensive Income (Loss)
The Company’s functional currency and reporting currency is the United States dollar. BBL’s functional currency is the Australian dollar (AUD). Assets and liabilities are translated at the exchange rate in effect at the balance sheet date. Expenses are translated at the average rate of exchange prevailing during the reporting period. Equity transactions are translated at each historical transaction date spot rate. Translation adjustments arising from the use of different exchange rates from period to period are included as a component of stockholders’ equity as “Accumulated other comprehensive loss.” Gains and losses resulting from foreign currency translation are included in the consolidated statements of operations and comprehensive loss as other comprehensive income (loss).
August 2023 Capital Raise
On August 11, 2023 we closed an underwritten public offering in which we sold 875,949 shares of common stock, 15,126,226 pre-funded warrants to purchase 15,126,226 shares of common stock, and 16,002,175 common warrants to purchase up to 16,002,175 shares of common stock. The pre-funded warrants were immediately exercisable until exercised in full at an exercise price of $0.0001 per share of common stock. The common warrants were immediately exercisable at a price per share of common stock of $3.86 and expire on the fifth anniversary of such initial exercisable date. The combined purchase price for each share of common stock and accompanying common warrant was $1.93, which was allocated as $1.9299 per share of common stock and
25
Table of Contents
$0.0001 per common warrant. Each pre-funded warrant was sold together with one common warrant at a combined price of $1.9299, which was allocated as $1.9298 per pre-funded warrant and $0.0001 per common warrant. In addition, the Company granted the underwriter a 30-day option to purchase up to 2,331,606 additional shares of common stock and/or up to 2,331,606 additional common warrants. The underwriter partially exercised this option and purchased 458,134 additional shares of common stock and 458,134 additional common warrants. Net proceeds from the offering, including the impact of the underwriter’s partial exercise of its option and net of underwriting discounts, commissions, and other offering expenses, totaled $27.9 million.
The Company has outstanding Series 2 warrants (the “Series 2 Warrants”) which are currently exercisable into 101,537 shares of common stock after giving effect to the Reverse Stock Split and exercises as of June 30, 2025. The Series 2 Warrants contain an exercise price adjustment mechanism providing that certain issuances of common stock (or common stock equivalents) if made at a price lower than the existing exercise price of $11.22 of such Series 2 Warrants, would reset the exercise price to such lower price. As a result of the August 11, 2023 public offering, the exercise price of the Series 2 Warrants has been automatically reset as of the closing time of such public offering to $1.9299.
April 2024 Capital Raise
On April 22, 2024, we closed a private investment in public equity (PIPE) financing in which we sold 5,749,152 shares of common stock at a price per share of $4.80 and, in lieu of shares of common stock, pre-funded warrants to purchase up to an aggregate of 2,584,239 shares of common stock at a price per pre-funded warrant of $4.7999, to certain accredited institutional investors. The pre-funded warrants were immediately exercisable until exercised in full at an exercise price of $0.0001 per share of common stock. Gross proceeds from the financing totaled $40.0 million. Net proceeds, net of commissions and other offering expenses, totaled approximately $37.1 million.
March 2025 Capital Raise
On March 25, 2025, we entered into an Underwriting Agreement with Leerink Partners LLC and TD Securities (USA) LLC, as representatives of the several underwriters named therein, pursuant to which we agreed to issue and sell, in an underwritten offering by us (the “Underwritten Offering”), (i) 1,143,000 shares of our common stock at a purchase price to investors of $13.00 per share, and (ii) pre-funded warrants to purchase 300,000 shares of Common Stock at an exercise price of $0.0001 per share at a purchase price to investors of $12.999 per warrant, and a Securities Purchase Agreement with Averill Master Fund, Ltd. and Averill Madison Master Fund, Ltd. (together, the “Purchasers”), pursuant to which we agreed to issue and sell to the Purchasers an aggregate of 900,000 shares of Common Stock at a purchase price of $13.00 per share in a registered direct offering (the “Direct Offering,” and together with the Underwritten Offering, the “Offerings”), the same price per share as the offering price in the Underwritten Offering. We received gross proceeds of approximately $30.5 million and net proceeds of approximately $28.2 million from the Offerings.
Employees
As of June 30, 2025, we had 19 full-time employees, one of whom has an MD, PhD, five have a PhD, four have a Master’s Degree, two have a biotechnology certificate, and one has an MBA, for a total of 13 with post-graduate degrees. Of these full-time employees, 14 are engaged in research and development activities and five are engaged in finance, legal, human resources, facilities and general management. None of our employees are represented by any labor union. All employees are in the United States.
Corporate Information
We were incorporated as a Delaware corporation on November 22, 2019, and completed our re-domiciliation from Australia to the United States of America on April 15, 2020. Our predecessor, Benitec Limited, was incorporated under the laws of Australia in 1995. Our principal executive offices are located at 3940 Trust Way, Hayward, California 94545.
26
Table of Contents
Our common stock began trading on The Nasdaq Capital Market, or Nasdaq, at the start of trading on the Implementation Date under the symbol “BNTC.”
Reverse Stock Split
On July 26, 2023, the Company effected a 1-for-17 reverse stock split (the “Reverse Stock Split”) of its common stock. In accordance with the Reverse Stock Split, 17 pre-split shares of the Company’s common stock were automatically converted into one issued and outstanding post-split share. Proportional adjustments were also made to all outstanding stock options, pre-funded warrants, and common warrants in accordance with their respective terms. The Reverse Stock Split did not change the par value of the Company’s common stock or the authorized number of shares. No fractional shares were issued in connection with the Reverse Stock Split. All fractional shares were rounded up to the nearest whole share with respect to outstanding shares of common stock. All share and earnings per share amounts presented in this Form 10-K reflect the impact of this reverse split.
Available Information
Our telephone number is (510) 780-0819, and our Internet website is www.benitec.com. The information on, or that can be accessed through, our website is not part of this Annual Report on Form 10-K and is not incorporated by reference herein.
27
Table of Contents
| Item 1A. | Risk Factors. |
Investing in our securities involves a high degree of risk. You should carefully consider the risks and uncertainties described below together with all of the other information contained in this Annual Report, including our consolidated financial statements and the related notes, before deciding to invest in our securities. The risks and uncertainties described below and in the documents incorporated by reference herein are not the only ones we face. Additional risks and uncertainties that we are unaware of, or that we currently believe are not material, may also become important factors that affect us. If any of the following risks actually occurs, our business, financial condition, results of operations and prospects could be materially and adversely affected, and the trading price of our common stock could decline.
Risk Factor Summary
The following is a summary of the risks and uncertainties that could cause our business, financial condition or operating results to be harmed. We encourage you to carefully review the full risk factors contained in this report in their entirety for additional information regarding these risks and uncertainties.
| • | We have incurred significant losses since inception and anticipate that we will continue to incur losses for the foreseeable future. If we are unable to achieve or sustain profitability, the market value of our common stock will likely decline; |
| • | We have never generated any revenue from product sales and may never be profitable; |
| • | We will need to continue our efforts to raise additional funding, which may not be available on acceptable terms, or at all. Failure to obtain capital when needed may negatively impact our ability to continue as a going concern; |
| • | Our product candidates are based on ddRNAi and silence and replace technology. Currently, no product candidates utilizing ddRNAi technology or silence and replace technology have been approved for commercial sale and our approach to the development of ddRNAi technology and silence and replace technology may not result in safe, effective or marketable products; |
| • | We are early in our product development efforts and our current product candidate is in early clinical stage. We may not be able to obtain regulatory approvals for the commercialization of our product candidates; |
| • | Issues that may impact delivery of our therapeutics to the cell could adversely affect or limit our ability to develop and commercialize product candidates; |
| • | We face competition from entities that have developed or may develop product candidates for our target disease indications, including companies developing novel treatments and technology platforms based on modalities and technology similar to ours; and |
| • | If we are unable to obtain or protect sufficient intellectual property rights related to our product candidates, we may not be able to obtain exclusivity for our product candidates or prevent others from developing similar competitive products. |
Risks Related to Our Financial Condition, Capital Requirements
We have incurred significant losses since inception and anticipate that we will continue to incur losses for the foreseeable future. If we are unable to achieve or sustain profitability, the market value of our common stock will likely decline.
As of June 30, 2025, we had accumulated losses of $228.2 million. We have devoted most of our financial resources to research and development, including our clinical and preclinical development activities. To date, we and our predecessor have financed our operations primarily through the issuance of equity securities, research
28
Table of Contents
and development grants from the Australian government and payments from our collaboration partners. We do not expect to generate any significant revenue for the foreseeable future, and we expect to incur significant operating losses for the foreseeable future due to the cost of research and development, preclinical studies and clinical trials and the regulatory approval process for product candidates. The amount of our future net losses is uncertain and will depend, in part, on the rate of our future expenditures. Our ability to continue operations will depend on, among other things, our ability to obtain funding through equity or debt financings, strategic collaborations or additional grants.
We expect to continue to incur significant expenses, and we may incur operating losses for the foreseeable future. We anticipate that our expenses will increase substantially if and as we:
| • | continue our research and preclinical development of our product candidates; |
| • | expand the scope of our current preclinical and clinical studies for our product candidates or initiate clinical, additional preclinical or other studies for product candidates; |
| • | seek regulatory and marketing approvals for any of our product candidates that successfully complete clinical trials; |
| • | further develop the manufacturing process for our product candidates; |
| • | change or add additional manufacturers or suppliers; |
| • | seek to identify and validate additional product candidates; |
| • | acquire or in-license other product candidates and technologies, which may or may not include those related to our ddRNAi technology and delivery vectors for our therapeutic candidates; |
| • | maintain, protect and expand our intellectual property portfolio; |
| • | create additional infrastructure to support our operations as a public company in the United States and our product development and future commercialization efforts; and |
| • | experience any delays or encounter issues with any of the above. |
The net losses we incur may fluctuate significantly from quarter to quarter and year to year, such that a period-to-period comparison of our results of operations may not be a good indication of our future performance. In any particular quarter or quarters, our operating results could be below the expectations of securities analysts or investors, which could cause the price of our common stock to decline.
We have never generated any revenue from product sales and may never be profitable.
Our ability to generate significant revenue and achieve profitability depends on our ability to, alone or with strategic collaboration partners, successfully complete the development of and obtain the regulatory approvals for our product candidates, to manufacture sufficient supply of our product candidates, to establish a sales and marketing organization or suitable third-party alternative for the marketing of any approved products and to successfully commercialize any approved products on commercially reasonable terms.
Our ability to generate future revenues from commercializing product candidates depends heavily on our success in:
| • | establishing proof of concept in preclinical studies and clinical trials for our product candidates; |
| • | successfully initiating and completing clinical trials of our product candidates; |
| • | obtaining regulatory and marketing approvals for product candidates for which we complete clinical trials; |
| • | maintaining, protecting and expanding our intellectual property portfolio, and avoiding infringing on intellectual property of third parties; |
29
Table of Contents
| • | establishing and maintaining successful licenses, collaborations and alliances with third parties; |
| • | developing a sustainable, scalable, reproducible and transferable manufacturing process for our product candidates; |
| • | establishing and maintaining supply and manufacturing relationships with third parties that can provide products and services adequate, in amount and quality, to support clinical development and commercialization of our product candidates, if approved; |
| • | launching and commercializing any product candidates for which we obtain regulatory and marketing approval, either by collaborating with a partner or, if launched independently, by establishing a sales, marketing and distribution infrastructure; |
| • | obtaining market acceptance of any product candidates that receive regulatory approval as viable treatment options; |
| • | obtaining favorable coverage and reimbursement rates for our products from third-party payers; |
| • | addressing any competing technological and market developments; |
| • | identifying and validating new product candidates; and |
| • | negotiating favorable terms in any collaboration, licensing or other arrangements into which we may enter. |
The process of developing product candidates for ddRNAi-based and antisense RNA-based therapeutics contains a number of inherent risks and uncertainties. For example, with regard to ddRNAi, it may not be possible to identify a target region of a disease-associated gene that has not been previously identified and/or patented by others, resulting in restrictions on freedom to operate for that target sequence. Silencing the target gene may not ultimately result in curing the disease as there may be more factors contributing to the development of the disease than the target gene. Silencing the target gene using ddRNAi may lead to short-term or long-term adverse effects that were not predicted or observed in preclinical studies. The delivery of the DNA construct to the target cells may not be possible, or complete or adequate to provide sufficient therapeutic benefit.
Even if one or more of our product candidates is approved for commercial sale, we may incur significant costs associated with commercializing any approved product candidate. As one example, our expenses could increase beyond expectations if we are required by the United States Food and Drug Administration (“FDA”) or other regulatory agencies, domestic or foreign, to perform clinical and other studies in addition to those that we currently anticipate. Even if we are able to generate revenues from the sale of any approved products, we may not become profitable and may need to obtain additional funding to continue operations, which could have an adverse effect on our business, financial condition, results of operations and prospects.
We will need to continue our efforts to raise additional funding, which may not be available on acceptable terms, or at all. Failure to obtain capital when needed may negatively impact our ability to continue as a going concern.
Because the length of time and activities associated with successful development of our product candidates is highly uncertain, we are unable to estimate the actual funds we will require for development and any approved marketing and commercialization activities. In any event, we will require additional capital to obtain regulatory approval for our product candidates and to commercialize any product candidates that receive regulatory approval. We estimate that our cash and cash equivalents will be sufficient to fund the Company’s operations for at least the next twelve months after the date that this Annual Report is filed.
Any additional fundraising efforts may divert our management from their day-to-day activities, which may compromise our ability to develop and commercialize our product candidates. In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all. Moreover, the
30
Table of Contents
terms of any financing may adversely affect the holdings or the rights of our stockholders, and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our common stock to decline. If we incur indebtedness we may be required to agree to restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could compromise our ability to conduct our business. We could also seek financing through arrangements with collaborative partners at an earlier stage than would otherwise be desirable and we may be required to relinquish rights to some or all of our technologies or product candidates or otherwise agree to terms unfavorable to us.
If we are unable to obtain funding on a timely basis or on acceptable terms, we may be required to significantly curtail, delay or discontinue one or more of our research or development programs or the commercialization of any approved product candidates.
We will continue to seek to raise additional working capital through public equity, private equity or debt financings. If we fail to raise additional working capital, or do so on commercially unfavorable terms, it may materially and adversely affect our business, prospects, financial condition and results of operations, and we may be unable to continue as a going concern. Future reports from our independent registered public accounting firm may also contain statements expressing substantial doubt about our ability to continue as a going concern. If we seek additional financing to fund our business activities in the future and there remains substantial doubt about our ability to continue as a going concern, investors or other financing sources may be unwilling to provide additional funding to us on commercially reasonable terms, if at all.
Risks Related to the Product Development and Regulatory Approval of Our Product Candidates
Our product candidates are based on ddRNAi and silence and replace technology. Currently, no product candidates utilizing ddRNAi technology or silence and replace technology have been approved for commercial sale and our approach to the development of ddRNAi technology and silence and replace technology may not result in safe, effective or marketable products.
We have concentrated our product research and development efforts on our ddRNAi technology and silence and replace technology, and our future success depends on successful clinical development of these technologies. We plan to progress our product candidates using our ddRNAi technology and our silence and replace technology for the treatment of the life-threatening conditions of OPMD.
The scientific research that forms the basis of our efforts to develop product candidates is based on the therapeutic use of ddRNAi, and the identification, optimization and delivery of ddRNAi-based product candidates is relatively new. The scientific evidence to support the feasibility of successfully developing therapeutic treatments based on ddRNAi is preliminary and limited. There can be no assurance that any development and technical problems we experience in the future will not cause significant delays or unanticipated costs, or that such development problems can be solved. We may be unable to reach agreement on favorable terms, or at all, with providers of vectors needed to optimize delivery of our product candidates to target disease cells and we may also experience unanticipated problems or delays in expanding our manufacturing capacity or transferring our manufacturing process to commercial partners, any of which may prevent us from completing our preclinical trials, initiating clinical trials or commercializing our products on a timely or profitable basis, if at all.
Only a few product candidates based on ddRNAi have been tested in either animals or humans, and a number of clinical trials conducted by other companies using other forms of RNAi technologies have not been successful. We may discover that application of ddRNAi does not possess properties required for a therapeutic benefit, such as the ability to continually express shRNAs for the period of time required to be maximally effective or the ability of viral vectors or other technologies to effectively deliver ddRNAi constructs to target cells in therapeutically relevant concentrations. In addition, application of ddRNAi-based products in humans may result in safety issues. We currently have only limited data, and no conclusive evidence, to suggest that we can effectively produce effective therapeutic treatments using our ddRNAi technology.
31
Table of Contents
We are early in our product development efforts and our current product candidates are still in preclinical development. We may not be able to obtain regulatory approvals for the commercialization of our product candidates.
The research, testing, manufacturing, labeling, approval, selling, marketing and distribution of biologics is subject to extensive regulation by the FDA and other regulatory authorities, and these regulations differ from country to country. We do not have any products on the market and are early in our development efforts. All of our ddRNAi product candidates and our silence and replace product candidates are in preclinical development. Our current product candidates are subject to the risks of failure typical for development of biologics. The development and approval process is expensive and can take many years to complete, and its outcome is inherently uncertain. In addition, the outcome of preclinical testing and early clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results.
We have not submitted a marketing application, or received marketing approval, for any of our product candidates and will not submit any applications for marketing approval for several years. We have limited experience in conducting and managing clinical trials necessary to obtain regulatory approvals, including marketing approval by the FDA. To receive marketing approval, we must, among other things, demonstrate with evidence from clinical trials that the product candidate is both safe and effective for each indication for which approval is sought, and failure can occur in any stage of development. Satisfaction of the marketing approval requirements typically takes several years and the time needed to satisfy them may vary substantially, based on the type, complexity and novelty of the biopharmaceutical product. We cannot predict if or when we might receive regulatory approvals for any of our product candidates currently under development.
The FDA and foreign regulatory authorities also have substantial discretion in the biopharmaceutical product approval process. The numbers, types and sizes of preclinical studies and clinical trials that will be required for regulatory approval varies depending on the product candidate, the disease or condition that the product candidate is designed to address and the regulations applicable to any particular product candidate. Approval policies, regulations or the type and amount of clinical data necessary to gain marketing approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions, and there may be varying interpretations of data obtained from preclinical studies or clinical trials, any of which may cause delays or limitations in the marketing approval or the decision not to approve an application. Regulatory agencies can delay, limit or deny marketing approval of a product candidate for many reasons, including:
| • | the FDA or comparable foreign regulatory authorities may disagree with the design or implementation of any future clinical trials; |
| • | we may be unable to demonstrate to the satisfaction of the FDA or comparable foreign regulatory authorities that a product candidate is safe and effective for its proposed indication; |
| • | the results of clinical trials may not meet the level of statistical or clinical significance required by the FDA or comparable foreign regulatory authorities for approval; |
| • | the patients recruited for a particular clinical program may not be sufficiently broad or representative to assure safety and effectiveness in the full population for which we seek approval; |
| • | the results of clinical trials may not confirm the positive results from earlier preclinical studies or clinical trials; |
| • | we may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks; |
| • | the FDA or comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials; |
| • | the data collected from clinical trials of our product candidates may not be sufficient to the satisfaction of FDA or comparable foreign regulatory authorities to support the submission of a biologics license application, or BLA, or other comparable submission in foreign jurisdictions or to obtain regulatory approval in the United States or elsewhere; |
32
Table of Contents
| • | the FDA or comparable foreign regulatory authorities may only agree to approve a product candidate under conditions that are so restrictive that the product is not commercially viable; |
| • | regulatory agencies might not approve or might require changes to our manufacturing processes or facilities; or |
| • | regulatory agencies may change their approval policies or adopt new regulations in a manner rendering our clinical data insufficient for approval. |
Any delay in obtaining or failure to obtain required approvals could materially and adversely affect our ability to generate revenue from the particular product candidate, which likely would result in significant harm to our financial position and adversely impact the price of our common stock. Furthermore, any regulatory approval to market a product may be subject to limitations on the indicated uses for which we may market the product. These limitations may limit the size of the market for the product.
We are not permitted to market our product candidates in the United States or in other countries until we receive approval of a BLA from the FDA or marketing approval from applicable regulatory authorities outside the United States. Obtaining approval of a BLA can be a lengthy, expensive and uncertain process. If we fail to obtain FDA approval to market our product candidates, we will be unable to sell our product candidates in the United States, which will significantly impair our ability to generate revenues. In addition, failure to comply with FDA and non-U.S. regulatory requirements may, either before or after product approval, if any, subject us to administrative or judicially imposed sanctions, including:
| • | restrictions on our ability to conduct clinical trials, including full or partial clinical holds on ongoing or planned trials; |
| • | restrictions on the products, manufacturers, or manufacturing process; |
| • | warning letters; |
| • | civil and criminal penalties; |
| • | injunctions; |
| • | suspension or withdrawal of regulatory approvals; |
| • | product seizures, detentions or import bans; |
| • | voluntary or mandatory product recalls and publicity requirements; |
| • | total or partial suspension of production; |
| • | imposition of restrictions on operations, including costly new manufacturing requirements; and |
| • | refusal to approve pending BLAs or supplements to approved BLAs. |
Even if we do receive regulatory approval to market a product candidate, any such approval may be subject to limitations on the indicated uses for which we may market the product. It is possible that none of our existing product candidates or any product candidates we may seek to develop in the future will ever obtain the appropriate regulatory approvals necessary for us or our collaborators to commence product sales. Any delay in obtaining, or an inability to obtain, applicable regulatory approvals would prevent us from commercializing our product candidates, generating revenues and achieving and sustaining profitability.
Because our ddRNAi and silence and replace product candidates are considered gene therapies, it is difficult to predict the time and cost of product candidate development as well as subsequently obtaining regulatory approval.
The clinical trial requirements of the FDA and comparable foreign regulatory authorities and the criteria these regulators use to determine the safety and efficacy of a product candidate vary substantially according to the
33
Table of Contents
type, complexity, novelty and intended use and market of such product candidates. The regulatory approval process for novel product candidates such as ours can be more expensive and take longer than for other pharmaceutical product candidates. The FDA and comparable foreign regulatory authorities have relatively limited experience with ddRNAi-based and silence and replace- based therapeutics, which makes it difficult to determine how long it will take or how much it will cost to obtain regulatory approvals for our product candidates in the United States or other countries. We and our current collaborators, or any future collaborators, may never receive approval to market and commercialize any product candidate. Even if we or a collaborator obtain regulatory approval, the approval may be for disease indications or patient populations that are not as broad as we intended or desired and may require labeling that includes significant use or distribution restrictions or safety warnings.
Regulatory requirements governing gene and cell therapy products have changed frequently and may continue to change in the future. Also, before a clinical trial can begin at any institution, that institution’s institutional review board, or IRB, and its institutional biosafety committee, or IBC, if it has one, have to review the proposed clinical trial to assess the safety of the trial. In addition, adverse developments in clinical trials of gene therapy products conducted by others may cause the FDA or comparable foreign regulatory bodies to change the requirements for approval of any of our product candidates.
Delay or failure to obtain, or unexpected costs in obtaining, the regulatory approval necessary to bring a potential product to market would delay or prevent us from commercializing our product candidates, generating revenues and achieving and sustaining profitability.
Positive results from preclinical studies of our product candidates are not necessarily predictive of the results of our planned clinical trials of our product candidates.
Positive results in preclinical proof of concept and animal studies of our product candidates may not result in positive results in clinical trials in humans. Many companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in clinical trials after achieving positive results in preclinical development or early stage clinical trials, and we cannot be certain that we will not face similar setbacks. These setbacks have been caused by, among other things, preclinical findings made while clinical trials were underway or safety or efficacy observations made in clinical trials, including adverse events. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that believed their product candidates performed satisfactorily in preclinical studies and clinical trials nonetheless failed to obtain FDA or other regulatory authority approval. If we fail to produce positive results in our clinical trials of our product candidates, the development timeline and regulatory approval and commercialization prospects for our product candidates, and, correspondingly, our business and financial prospects, would be negatively impacted.
Issues that may impact delivery of our therapeutics to the cell could adversely affect or limit our ability to develop and commercialize product candidates.
Successful clinical development of ddRNAi-based therapeutics and silence and replace-based therapeutics is largely dependent on using the appropriate delivery methodologies, including viral vectors, to obtain therapeutically relevant concentrations of the DNA constructs that express the shRNAs and engineered transgenes in the appropriate target cells. To develop effective product candidates, we will need to license delivery technologies from third parties or develop delivery technologies with research collaborators. Although delivery technologies, including AAV vectors, have been identified and are well defined for specific tissue types, we continue to seek vectors with ideal delivery properties for indications we are pursuing, including OPMD.
The tissue tropism and other physical properties of AAV vectors are limited and may not be effective for other product candidates or delivery into a wide array of tissues types. AAV vectors can also trigger immune responses in some patients, and those patients will not derive clinical benefit from administration of a product candidate unless steps are taken to clinically address the issue. If we or our collaborators are not successful in identifying effective delivery methodologies to achieve a therapeutically relevant concentration for our product candidates in
34
Table of Contents
the target tissues, we may not succeed in developing marketable products. In addition, if we are unable to reach agreement on favorable terms, or at all, with providers of any effective vectors that we do identify, we may not succeed in completing our clinical trials or commercializing our products on a timely or profitable basis, if at all.
We may find it difficult to enroll patients in our clinical trials, and patients could discontinue their participation in our current and any future clinical trials, which could delay or prevent our clinical trials of our product candidates and make those trials more expensive to undertake.
Identifying and qualifying patients to participate in clinical trials of our product candidates is critical to our success. The timing of our clinical trials depends on the speed at which we can recruit patients to participate in testing our product candidates. Patients may be unwilling to participate in any clinical trials because of negative publicity from adverse events in the biotechnology, RNAi or gene therapy industries. Patients may be unavailable for other reasons, including competitive clinical trials for similar patient populations, and the timeline for recruiting patients, conducting trials and obtaining regulatory approval of potential products may be delayed. If we have difficulty enrolling a sufficient number of patients to conduct any future clinical trials as planned, we may need to delay, limit or discontinue those clinical trials. Clinical trial delays could result in increased costs, slower product development, setbacks in testing the safety and effectiveness of our technology or discontinuation of the clinical trials altogether.
We may not be able to identify, recruit and enroll a sufficient number of patients, or those with required or desired characteristics to achieve diversity in a trial, to complete any clinical trials in a timely manner. Patient enrollment is affected by factors including:
| • | finding and diagnosing patients; |
| • | severity of the disease under investigation; |
| • | design of the clinical trial protocol; |
| • | size and nature of the patient population; |
| • | eligibility criteria for the trial in question; |
| • | perceived risks and benefits of the product candidate under study; |
| • | proximity and availability of clinical trial sites for prospective patients; |
| • | availability of competing therapies and clinical trials; |
| • | clinicians’ and patients’ perceptions of the potential advantages of the product being studied in relation to other available therapies, including any new products that may be approved for the indications we are investigating; |
| • | patient referral practices of physicians; and |
| • | ability to monitor patients adequately during and after treatment. |
We or any potential collaborators plan to seek initial marketing approval for our product candidates in the United States and other regions and countries. We may not be able to initiate or continue clinical trials in a timely manner if we cannot enroll a sufficient number of eligible patients to participate in the clinical trials required by the FDA or comparable foreign regulatory agencies. Our ability to successfully initiate, enroll and complete a clinical trial in any foreign country is subject to numerous risks unique to conducting business in foreign countries, including:
| • | difficulty in establishing or managing relationships with contract research organizations, or CROs, clinical sites and physicians; |
| • | different standards for the conduct of clinical trials; |
35
Table of Contents
| • | our inability to locate and engage qualified local consultants, physicians and partners; and |
| • | the potential burden of complying with a variety of foreign laws, medical standards and regulatory requirements, including the regulation of biopharmaceutical and biotechnology products and treatments. |
In addition, patients enrolled in clinical trials may discontinue their participation at any time during the trial as a result of a number of factors, including experiencing adverse events, which may or may not be judged related to our product candidates under evaluation. The discontinuation of patients in any one of our trials may cause us to delay or discontinue our clinical trial, or cause the results from that trial not to be positive or sufficient to support either partnering with a pharmaceutical or biotechnology company to further develop and commercialize the product candidate or filing for regulatory approval of the product candidate.
We may encounter substantial delays in our clinical trials or we may fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory authorities.
Before obtaining marketing approval from regulatory authorities for the sale of any of our product candidates, we must conduct extensive clinical trials to demonstrate the safety and efficacy of the product candidates in humans. Clinical trials are expensive and time-consuming, and their outcome is uncertain. We cannot guarantee that any particular clinical trials will be initiated or conducted as planned or completed on schedule, if at all. A failure of one or more clinical trials can occur at any stage of testing. Events that may prevent successful or timely completion of clinical development include:
| • | inability to generate sufficient preclinical, toxicology or other data to support the initiation of human clinical trials; |
| • | delays in reaching consensus with regulatory agencies on trial design; |
| • | identifying, recruiting and training suitable clinical investigators; |
| • | delays in reaching agreement on acceptable terms with prospective CROs and clinical trial sites; |
| • | delays in obtaining required IRB or IBC approval at each clinical trial site; |
| • | delays in recruiting suitable patients to participate in our clinical trials; |
| • | imposition of a clinical hold by regulatory agencies, including after an inspection of our clinical trial operations or trial sites; |
| • | failure by our CROs, other third parties or us to adhere to clinical trial requirements; |
| • | failure to perform in accordance with the FDA’s good clinical practices, or GCP, or applicable regulatory requirements in other countries; |
| • | inability to manufacture, test, release, import or export for use sufficient quantities of our product candidates for use in clinical trials; |
| • | failure to manufacture our product candidate in accordance with cGMP requirements or applicable regulatory guidelines in other countries; |
| • | delays in the testing, validation and manufacturing of our product candidates; |
| • | delays in the delivery of our product candidates to the clinical trial sites; |
| • | delays in having patients complete participation in a trial or return for post-treatment follow-up; |
| • | clinical trial sites dropping out of a trial; |
| • | occurrence of serious adverse events associated with the product candidate that are viewed to outweigh its potential benefits; |
36
Table of Contents
| • | changes in regulatory requirements and guidance that require amending or submitting new clinical trial protocols; |
| • | clinical trials of our product candidates may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical trials or discontinue product development programs. |
Further, a clinical trial may be suspended or discontinued by us, our collaborators, the IRBs or the IBCs at the sites in which such trials are being conducted, the data safety monitoring board, or DSMB, for such trial, or by the FDA or comparable foreign regulatory authorities due to a number of factors, including the imposition of a clinical hold or termination of a trial due to failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, unforeseen safety issues or adverse side effects of our product candidate, or a product candidate from another company that shares similar properties, failure to demonstrate adequate benefit from using a product candidate, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. If we experience discontinuation of, or delays in the completion of, any clinical trial of our product candidates, the commercial prospects of our product candidates may be harmed, and our ability to generate product revenues from any of these product candidates may be eliminated or delayed. Furthermore, many of the factors that lead to a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
In addition, if we or our third-party collaborators make significant manufacturing or formulation changes to our product candidates, we or they may need to conduct additional studies to bridge the modified product candidates to earlier versions to ensure comparability, safety and efficacy of the two different product candidates.
Any inability to successfully complete preclinical and clinical development could result in additional costs to us or impair our ability to commercialize our programs and product candidates. Clinical trial delays could also shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do, which could impair our ability to successfully commercialize our product candidates.
If the results of our clinical trials are inconclusive or if there are safety concerns or adverse events associated with our product candidates, we may:
| • | fail to obtain, or be delayed in obtaining, marketing approval for our product candidates; |
| • | obtain approval for indications or patient populations that are not as broad as intended or desired; |
| • | obtain approval with labeling that includes significant use or distribution restrictions or safety warnings; |
| • | need to change the way the product is administered; |
| • | be required to perform additional clinical trials to support approval or be subject to additional post- marketing testing requirements; |
| • | have regulatory authorities withdraw their marketing approval of the product after granting it; |
| • | have regulatory authorities impose restrictions on distribution of the product in the form of a risk evaluation and mitigation strategy, or REMS, or modified REMS, that limit our ability to commercialize the product; |
| • | be subject to the addition of labeling statements, such as warnings or contraindications; |
| • | be sued and held liable for harm caused to patients; or |
| • | experience damage to our reputation. |
Any of these events could prevent us from achieving or maintaining market acceptance of our product candidates and impair our ability to commercialize our product candidates.
37
Table of Contents
In addition, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and may receive cash or equity compensation in connection with such services. If these relationships and any related compensation result in perceived or actual conflicts of interest, or the FDA concludes that the financial relationship may have affected the interpretation of any particular study, the integrity of the data generated at the applicable clinical trial site may be questioned and the utility of the clinical trial itself may be jeopardized, which could result in the delay or rejection of any BLA we submit to the FDA or any comparable foreign regulating authorities. Any such delay or rejection could prevent us from commercializing our product candidates.
Our product candidates and the process for administering our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following any potential marketing approval.
Treatment with our product candidates may produce undesirable side effects or adverse reactions or events. For example, the AAV vector and related capsid protein, which we are currently using to deliver many of our ddRNAi and silence and replace product candidates, could cause adverse immunological side effects due to preexisting and/or recall responses to the naturally occurring virus from which the vector is engineered, or to the DNA construct product itself. These responses may also interfere with therapeutic efficacy if not identified and managed optimally. Preexisting immune responses to AAV manifesting as neutralizing antibodies are common within the general population and may be a limitation to the enrollment of patients in gene therapy clinical trials using AAV vectors, the successful use of AAV vectors in gene therapy clinical trials and the market acceptance of product candidates, if approved, that are delivered using AAV vectors. Patients with neutralizing antibodies to AAV will not derive clinical benefit from administration of such a product candidate unless steps are taken to clinically address the issue and those treatments themselves may cause adverse effects. In previous clinical trials undertaken by other companies involving systemic administration of AAV viral vectors for gene therapy, some subjects experienced adverse events, including the development of a negative T cell response against the AAV capsid protein. If our vectors cause similar adverse events, we may be required to delay or discontinue further clinical development of our product candidates. It is also possible that we may discover new adverse events related to AAV or other vectors, which could potentially enhance the risk to patients who use our product candidates delivered with that vector. Additionally, the procedure used to administer the treatment could result in undesirable health effects.
If any such adverse events occur, our clinical trials could be suspended or discontinued and the FDA or comparable foreign regulatory authorities could order us to cease further development or deny approval of our product candidates for any or all targeted indications. The product-related side effects could affect patient recruitment or the ability of enrolled patients to complete the trial. If we elect or are required to delay, suspend or discontinue any clinical trial of any of our product candidates, the commercial prospects of such product candidates will be harmed and our ability to generate product revenues from any of these product candidates will be delayed or eliminated. Any of these occurrences may harm our business, financial condition and prospects significantly.
Additionally, if any of our biopharmaceutical product candidates receive marketing approval, the FDA could require us to adopt a REMS to ensure that the benefits of the product outweigh its risks, which may include, among other things, a medication guide outlining the risks of gene therapies for distribution to patients and a communication plan to patients and healthcare practitioners. Other elements to assure safe use in a mandated REMS could include, but are not limited to, restrictions upon distribution and prescribing, additional prescriber training, establishment of patient registries and other measures that could limit commercialization of the product. Comparable foreign regulating authorities might require adoption of measures similar to a REMS. Furthermore, if we or others later identify undesirable side effects caused by our product candidate, a number of potentially significant negative consequences could result, including:
| • | regulatory authorities may withdraw approvals of such product candidate; |
38
Table of Contents
| • | regulatory authorities may require additional warnings on the label; |
| • | we may be required to create a medication guide outlining the risks of such side effects for distribution to patients; |
| • | we may be required to change the way a product candidate is administered or conduct additional clinical trials; |
| • | we could be sued and held liable for harm caused to patients; and |
| • | our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of our product candidates and could significantly harm our business, prospects, financial condition and results of operations.
If we are unable to successfully develop related diagnostics for our therapeutic product candidates, or experience significant delays in doing so, we may not achieve marketing approval or realize the full commercial potential of our therapeutic product candidates.
We may develop related diagnostics for some of our therapeutic product candidates. Such related diagnostics are subject to regulation by the FDA and typically to comparable foreign regulatory authorities as medical devices and typically require separate regulatory approval or clearance prior to commercialization. Marketing approval or clearance of the diagnostic will require sufficient data to support its safety and efficacy. In addition, at least in some cases, the FDA and comparable foreign regulatory authorities may require the development and regulatory approval or clearance of a related diagnostic as a condition to approving our therapeutic product candidates. While we have some limited experience in developing diagnostics, we plan to rely in large part on third parties to perform these functions. We may seek to enter into arrangements with one or more third parties to create a related diagnostic for use with our current or future product candidates.
If we or any third parties that we engage to assist us are unable to successfully develop or obtain marketing approval or clearance for related diagnostics for our therapeutic product candidates, or experience delays in doing so:
| • | the development of relevant product candidates may be delayed or impaired altogether if we are unable to appropriately select patients for enrollment in our clinical trials; |
| • | our relevant therapeutic product candidate may not receive marketing approval if its effective use depends on a related diagnostic in the regulatory authority’s judgment; and |
| • | we may not realize the full commercial potential of any therapeutic product candidates that receive marketing approval if, among other reasons, we are unable to appropriately identify patients with the specific genetic alterations targeted by our therapeutic product candidates. |
If any of these events were to occur, our business would be harmed.
Even if we complete the necessary preclinical studies and clinical trials for a product candidate, we cannot predict when or if we will obtain regulatory approval to commercialize a product candidate or the approval may be for a narrower indication than we expect.
We cannot commercialize a product until the appropriate regulatory authorities have reviewed and approved the product candidate. Even if our product candidates demonstrate safety and efficacy in clinical trials, the regulatory agencies may not complete their review processes in a timely manner, or we may not be able to obtain regulatory approval. Additional delays may result if an FDA Advisory Committee or comparable foreign regulatory authority recommends non-approval or restrictions on approval. In addition, we may experience delays or rejections based upon additional government regulation from future legislation or administrative action, or
39
Table of Contents
changes in regulatory agency policy during the period of product development, clinical trials and the review process. Regulatory agencies may also approve a treatment candidate for fewer or more limited indications than requested, may not approve the price we intend to charge for our product candidate, may limit our ability to promote the product, may impose significant limitations upon the approval of the product, including, but not limited to, narrow indications, significant warnings, precautions or contraindications with respect to conditions of use, or may grant approval subject to the performance of post-marketing studies. In addition, regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of our product candidates. The FDA or comparable foreign regulatory authorities may impose a REMS or other conditions upon our approval that limit our ability to commercialize the product candidate.
Inadequate funding for the FDA and other government agencies could hinder their ability to hire and retain key leadership and other personnel, prevent new products and services from being developed or commercialized in a timely manner or otherwise prevent those agencies from performing normal business functions on which the operation of our business may rely, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of other government agencies on which our operations may rely, including those that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
Even if we obtain regulatory approval for a product candidate, our products will remain subject to regulatory scrutiny.
Even if we obtain regulatory approval in a jurisdiction, the regulatory authority may still impose significant restrictions on the indicated uses or marketing of our product candidates, or impose ongoing requirements for potentially costly post-approval studies or post-market surveillance. For example, the holder of an approved BLA is obligated to monitor and report to the FDA adverse events and any failure of a product to meet the specifications in the BLA. FDA guidance advises that patients treated with some types of gene therapy undergo follow-up observations for potential adverse events for as long as 15 years. The holder of an approved BLA must also submit new or supplemental applications and obtain FDA approval for certain changes to the approved product, product labeling or manufacturing process. Advertising and promotional materials must comply with FDA rules and are subject to FDA review, in addition to other potentially applicable foreign, federal and state laws.
In addition, product manufacturers and their establishments, products and applications are subject to payment of user fees/or and continual review and periodic inspections by the FDA and comparable foreign regulatory authorities for compliance with cGMP and comparable foreign requirements, and adherence to commitments made in the BLA. If we or a regulatory agency discover previously unknown problems with a product such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory agency may impose restrictions relative to that product or the manufacturing facility, including requiring recall or withdrawal of the product from the market or suspension of manufacturing.
If we fail to comply with applicable regulatory requirements following approval of any of our product candidates, a regulatory agency may:
| • | issue a warning letter asserting that we are in violation of the law; |
| • | seek an injunction or impose civil or criminal penalties or monetary fines; |
| • | suspend or withdraw regulatory approval; |
| • | suspend any ongoing clinical trials; |
40
Table of Contents
| • | refuse to permit government reimbursement of our product by government-sponsored third-party payers; |
| • | refuse to approve a pending BLA or supplements to a BLA submitted by us for other indications or new product candidates; |
| • | seize our product; or |
| • | refuse to allow us to enter into or continue supply contracts, including government contracts. |
Any government investigation of alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity. The occurrence of any event or penalty described above may inhibit our ability to commercialize our product candidates and generate revenues.
Even if we obtain and maintain approval for our product candidates from the FDA, we may never obtain approval for our product candidates outside of the United States, which would limit our market opportunities and adversely affect our business.
Approval of a product candidate in the United States by the FDA does not ensure approval of such product candidate by regulatory authorities in other countries or jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or by the FDA. Sales of our product candidates outside of the United States will be subject to foreign regulatory requirements governing clinical trials and marketing approval. Even if the FDA grants marketing approval for a product candidate, comparable regulatory authorities of foreign countries must also approve the manufacturing and marketing of the product candidates in those countries. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and greater than, those in the United States. Obtaining foreign regulatory approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our product candidates in certain countries.
Further, clinical trials conducted in one country may not be accepted by regulatory authorities in other countries. Regulatory approval of a product candidate in one country does not ensure approval in any other country, but a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory approval process in other countries. Also, regulatory approval for any of our product candidates may be withdrawn based on adverse events reported or regulatory decisions made in other countries. If we fail to comply with the regulatory requirements in international markets and/or fail to receive applicable marketing approvals, our target market will be reduced, our ability to realize the full market potential of our product candidates will be compromised and our business may be adversely affected.
Our future prospects may also depend on our ability to successfully develop a pipeline of additional product candidates beyond our initial product candidate, and we and our collaborators may not be successful in efforts to use our platform technologies to identify or discover additional product candidates.
The success of our business depends primarily upon our ability to identify, develop and commercialize products based on our platform technology. Our and our collaborators’ research methodology may be unsuccessful in identifying potential product candidates beyond our initial product candidate, BB-301, additional potential product candidates may not demonstrate the necessary preclinical outcomes to progress to clinical studies, or additional product candidates may be shown to have harmful side effects or may have other characteristics that may make the products unmarketable or unlikely to receive marketing approval.
If any of these events occur, we may be forced to discontinue our development efforts for a program or programs. Research programs to identify new product candidates require substantial technical, financial and human resources. We may focus our efforts and resources on potential programs or product candidates that ultimately prove to be unsuccessful.
41
Table of Contents
We may not be able to obtain orphan drug exclusivity, where relevant, in all markets for our product candidates.
Of our current pipeline product candidates, only our silence and replace therapeutic for the treatment of OPMD has been designated with orphan drug status. In January 2018, the FDA granted such designation after our candidate for the treatment of OPMD had been designated an orphan drug in January 2017 by the European Commission. Regulatory authorities in some jurisdictions, including the United States, may designate drugs or biological products for relatively small patient populations as orphan drugs. Under the Orphan Drug Act, the FDA may designate a product as an orphan drug if it is a product intended to treat a “rare disease or condition”, which is generally defined as any disease or condition which affects less than 200,000 individuals in the United States. The FDA may also designate a product as an orphan drug if it is intended to treat a disease or condition which affects more than 200,000 individuals in the United States and there is no reasonable expectation that the cost of developing and making a drug or biological product available in the United States for this type of disease or condition will be recovered from sales of the product candidate. Generally, if a product with an orphan drug designation subsequently receives the first marketing approval for the indication for which it has such designation, the product is entitled to a period of marketing exclusivity, which precludes the FDA from approving another marketing application for the same drug for such indication for that time period. The applicable period is seven years in the United States. Orphan drug exclusivity may be lost if the FDA or the European Medicines Agency, or the EMA, determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition. While there is no guarantee, FDA orphan drug designation may provide a range of benefits, including a potential fast track process for clinical regulatory approval, potential tax credits for qualified clinical trials and an exemption from FDA application user fees.
Even if we obtain orphan drug exclusivity for a product in the United States or for additional products in the European Union, that exclusivity may not effectively protect the product from competition because different drugs can be approved for the same condition, and the same drug could be approved for a different condition. Even after an orphan drug is approved, the FDA can subsequently approve the same drug, made by a competitor, for the same condition if the FDA concludes that the competitive product is clinically superior in that it is shown to be safer, more effective or makes a major contribution to patient care. In the European Union, the EMA can approve a competitive product if the orphan drug no longer meets the criteria for orphan designation (including sufficient profitability), if the competitive product is safer, more effective or otherwise clinically superior, or if the orphan drug cannot be supplied in sufficient quantities.
Risks Related to Our Reliance on Third Parties
Our prospects for successful development and commercialization of our products are dependent to varying degrees upon the research, development, commercialization, and marketing efforts of our collaborators.
We rely on third parties for certain aspects of the research, development, commercialization and marketing of our current and any future product candidates. Other than as provided for in our collaboration agreements, we have no control over the resources, time and effort that our collaborators may devote to the development of product candidates. We are dependent on our collaborators to conduct some aspects of the research and development of each of our product candidates, and expect to need access to them to facilitate and/or to complete the regulatory process. We will likely rely on a pharmaceutical company for the successful marketing and commercialization of any such product candidates for which they/we receive approval, if any. There can be no guarantee at this stage that we will conclude a partnership with such a company on favorable terms, or at all, nor even if we do so, that success will be achieved.
Our ability to recognize revenues from successful potential collaborations may be impaired by multiple factors including:
| • | a collaborator may shift its priorities and resources away from our programs due to a change in business strategies, or a merger, acquisition, sale or downsizing of its company or business unit; |
42
Table of Contents
| • | a collaborator may cease development in an area that is the subject of a collaboration agreement; |
| • | a collaborator may change the success criteria for a particular program or product candidate in development, thereby delaying or ceasing development of such program or product candidate in development; |
| • | a collaborator with development or commercialization obligations may not commit sufficient financial or human resources to the development, marketing, distribution or sale of a product, or may otherwise fail in development or commercialization efforts; |
| • | a collaborator with manufacturing responsibilities may encounter regulatory, resource or quality issues and be unable to meet demand requirement; |
| • | a collaborator could independently develop, or develop with unrelated parties, products that compete directly or indirectly with our product candidates; |
| • | a collaborator may exercise its rights under the agreement to discontinue our collaboration; |
| • | a dispute may arise between us and a collaborator concerning the development or commercialization of a product candidate, resulting in a delay in milestones, royalty payments, or discontinuation of a program and possibly resulting in costly litigation or arbitration that may divert management attention and resources; |
| • | a collaborator may not adequately protect the intellectual property rights associated with a product candidate; |
| • | a collaborator may use our proprietary information or intellectual property in such a way as to expose us actual or threatened litigation from a third party, patent office proceedings or other risks that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential liability; and |
| • | a collaborator may own or co-own, or have a license to use, intellectual property rights associated with a product candidate that results from our collaborating with them, and in such cases, we would not have the exclusive right to commercialize such intellectual property rights. |
If our potential collaborators do not perform in the manner we expect or fulfill their responsibilities in a timely manner, or at all, the development, regulatory and commercialization process could be delayed or discontinued or otherwise be unsuccessful. Conflicts between us and our collaborators may arise. In the event of discontinuation of one or more of our collaboration agreements, it may become necessary for us to assume the responsibility for any such product candidates at our own expense or seek new collaborators. In that event, we likely would be required to limit the size and scope of one or more of our independent programs or increase our expenditures and seek additional funding, which may not be available on acceptable terms or at all, and our business may be harmed.
We rely on third parties to conduct our preclinical studies and clinical trials.
We do not have the ability to conduct all aspects of our preclinical testing or clinical trials ourselves. We are dependent on third parties to conduct the preclinical studies for our product candidates and to conduct clinical trials for our product candidates, and therefore the timing of the initiation and completion of these trials and studies is reliant on third parties and may occur at times substantially different from our estimates or expectations.
In the case of clinical trials, we rely on CROs and other third-party collaborators to conduct clinical trials in accordance with our clinical protocols and regulatory requirements. Our CROs, investigators and third-party collaborators play a significant role in the conduct of these trials and subsequent collection and analysis of data. There is no guarantee that any CROs, investigators or the other third-party collaborators on which we rely for
43
Table of Contents
administration and conduct of our clinical trials will devote adequate time and resources to such trials or perform as contractually required. If any of these third parties fails to meet expected deadlines, fails to adhere to our clinical protocols, fails to meet regulatory requirements or otherwise performs in a substandard manner, our clinical trials may be extended, delayed or terminated. If our clinical trial sites terminate for any reason, we may lose all of the information on subjects enrolled in any such clinical trials.
If we cannot contract with acceptable third parties on commercially reasonable terms, or if these third parties do not carry out their contractual duties, satisfy legal and regulatory requirements for the conduct of preclinical studies or clinical trials or meet expected deadlines, our clinical development programs could be delayed or discontinued.
In all events, we are responsible for ensuring that each of our preclinical studies, and our future clinical trials are conducted in accordance with the general investigational plan and protocols for the study or trial. The FDA requires clinical trials to be conducted in accordance with current GCP, including for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights and confidentiality of clinical trial participants are protected. Our reliance on third parties that we do not control does not relieve us of these responsibilities and requirements, and any failure to satisfy these responsibilities and requirements, whether caused by us or by third parties upon whom we rely, could have a material adverse effect on our business, financial condition, results of operations and prospects.
Because we rely on third-party manufacturing and supply partners, our supply of research and development, preclinical and clinical development materials may become limited or interrupted or may not be of satisfactory quantity or quality.
We rely on third-party supply and manufacturing partners to manufacture and supply the materials for our research and development and preclinical and clinical study supplies. We do not own manufacturing facilities or supply sources for such materials.
There can be no assurance that our supply of research and development, preclinical and clinical development biologics and other materials will not be limited, interrupted or restricted in certain geographic regions, be of satisfactory quality or continue to be available at acceptable prices. Replacement of a third-party manufacturer could require significant effort, cost and expertise because there may be a limited number of qualified replacements.
The manufacturing process for a product candidate is subject to FDA and foreign regulatory authority review. Suppliers and manufacturers must meet applicable manufacturing requirements and undergo rigorous facility and process validation tests required by regulatory authorities in order to comply with regulatory standards, such as cGMP. In the event that any of our suppliers or manufacturers fails to comply with such requirements or to perform its obligations to us in relation to quality, timing or otherwise, or if our supply of components or other materials becomes limited or interrupted for other reasons, we may be forced to manufacture the materials ourselves or enter into an agreement with another third party, which would be costly and delay any future clinical trials.
In some cases, the technical skills or technology required to manufacture our product candidates may be unique or proprietary to the original manufacturer and we may have difficulty, or there may be contractual restrictions prohibiting us from, transferring such skills or technology to another third party. These factors increase our reliance on our manufacturers and may require us to obtain a license from a manufacturer in order to have another third-party manufacture our product candidates.
We expect to continue to rely on third-party manufacturers for preclinical and clinical grade product candidates and if we receive regulatory approval for any product candidate. If we are unable to obtain or maintain third-party manufacturing for product candidates, or to do so on commercially reasonable terms, we may not be able to
44
Table of Contents
develop and commercialize our product candidates successfully. Our or a third party’s failure to execute on our manufacturing requirements could adversely affect our business in a number of ways, including:
| • | an inability to conduct necessary preclinical studies to progress our product candidates to clinical trials; |
| • | an inability to initiate or continue clinical trials of product candidates under development; |
| • | delay in submitting regulatory applications, or receiving regulatory approvals, for product candidates; |
| • | loss of the cooperation of a collaborator; |
| • | subjecting our product candidates to additional inspections by regulatory authorities; |
| • | requirements to cease distribution or to recall batches of our product candidates; and |
| • | in the event of approval to market and commercialize a product candidate, an inability to meet commercial demands for our products. |
Our third-party manufacturers may be subject to damage or interruption from, among other things, events such as pandemics, fire, natural or man-made disaster, power loss, telecommunications failure, unauthorized entry, computer viruses, denial-of-service attacks, acts of terrorism, human error, vandalism or sabotage, financial insolvency, bankruptcy and similar events.
We and our licensees or collaborators may disagree over our right to receive payments under any potential collaboration agreements with them, potentially resulting in costly litigation and loss of reputation.
Our ability to receive payments under any out-license and collaboration agreements depends on our ability to clearly delineate our rights under those agreements. We have licensed and may license additional portions of our intellectual property to our collaborators with the intent that our collaborators will develop product candidates based on our ddRNAi or other technology to address specific conditions. However, a collaborator may use our intellectual property without our permission, dispute our ownership of intellectual property rights, or argue that our intellectual property does not cover, or add value to, any product candidates they develop. If a dispute arises, it may result in costly patent office procedures and litigation, and our collaborator may refuse to pay us while the dispute is ongoing. Furthermore, regardless of any resort to legal action, a dispute with a collaborator over intellectual property rights may damage our relationship with that collaborator and may also harm our reputation in the industry. Even if we are entitled to payments from our collaborators, we may not actually receive these payments, or we may experience difficulties in collecting the payments to which we believe we are entitled. After our collaborators launch commercial products containing our licensed traits, we will need to rely on the good faith of our collaborators to report to us the sales they earn from these products and to accurately calculate the payments we are entitled to, a process that will involve complicated and difficult calculations. Although we seek to address these concerns in our license and collaboration agreements by reserving our right to audit financial records, such provisions may not be effective.
We have only limited experience in regulatory affairs and intend to rely on consultants and other third parties for regulatory matters, which may affect our ability or the time we require to obtain necessary regulatory approvals.
We have limited experience in filing and prosecuting the applications necessary to gain regulatory approvals for gene therapy or ddRNAi product candidates. Moreover, the product candidates that are likely to result from our development programs are based on novel technologies that have not been extensively tested in humans. The regulatory requirements governing these types of product candidates may be less well defined or more rigorous than for conventional products. As a result, we may experience a longer regulatory process in connection with obtaining regulatory approvals of any products that we develop. We intend to rely on independent consultants for purposes of our regulatory compliance and product development and approvals in the United States and elsewhere. Any failure by our consultants to properly advise us regarding, or properly perform tasks related to, regulatory compliance requirements could compromise our ability to develop and seek regulatory approval of our product candidates.
45
Table of Contents
Our reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed.
Because we rely on third parties to manufacture our product candidates, and because we collaborate with various organizations and academic institutions on the advancement of our technology and product candidates, we may, at times, share trade secrets with them. We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, collaborative research agreements, consulting agreements or other similar agreements with our collaborators, advisors, employees and consultants prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of the third parties to use or disclose our confidential information, such as trade secrets. Despite these contractual provisions, the need to share trade secrets and other confidential information increases the risk that such trade secrets become known by potential competitors, are inadvertently incorporated into the technology of others, or are disclosed or used in violation of these agreements. Given that our proprietary position is based, in part, on our know-how and trade secrets, discovery by a third party of our trade secrets or other unauthorized use or disclosure would impair our intellectual property rights and protections in our product candidates.
In addition, these agreements typically restrict the ability of our collaborators, advisors, employees and consultants to publish data potentially relating to our trade secrets. Our academic collaborators typically have rights to publish data, provided that we are notified in advance and may delay publication for a specified time in order to secure our intellectual property rights arising from the collaboration. In other cases, publication rights are controlled exclusively by us or we may share these rights with other parties. Despite our efforts to protect our trade secrets, our competitors may discover our trade secrets, either through breach of these agreements, independent development or publication of information including our trade secrets in cases where we do not have proprietary or otherwise protected rights at the time of publication.
Risks Related to Commercialization of Our Product Candidates
If we are unable to enter into agreements with third-party manufacturers on commercially reasonable terms, the commercialization of our product candidates may be adversely affected.
We intend to rely on third-party manufacturers for commercialization and production of commercial quantities of our product candidates. We may be unable to negotiate agreements with third-party manufacturers to support our commercialization activities on commercially reasonable terms.
We may encounter technical or scientific issues related to manufacturing or development that we may be unable to resolve in a timely manner or with available funds. If we are unable to engage manufacturing partners to produce our product candidates on a larger scale on reasonable terms, our commercialization efforts will be harmed.
If such third-party manufacturers are unable to produce the necessary quantities of our product candidates, or do so in compliance with cGMP or with pertinent foreign regulatory requirements, and within our planned time frame and cost parameters, the development and sales of our product candidates, if approved, may be impaired. In some jurisdictions, approval of the manufacturer may be required. There is no guarantee such approval can be obtained.
If we are unable to enter into agreements with third parties to commercialize our product candidates or establish sales and marketing capabilities to market and sell our product candidates, we may be unable to generate any revenues.
To successfully commercialize any product candidates that may be approved, we will need to develop our sales and marketing capabilities, either through our relationships with collaborators or our own. We may seek to enter into collaborations with other entities to utilize their marketing and distribution capabilities, but we may be unable to enter into marketing agreements on favorable terms, if at all. If our future collaborators do not commit
46
Table of Contents
sufficient resources to commercialize our future products, if any, and we are unable to develop the necessary marketing capabilities on our own, we will be unable to generate sufficient product revenue to sustain our business. The establishment and development of our own sales force or the establishment of a contract sales force to market any products we may develop will be expensive and time-consuming and could delay any product launch. We will be competing with many companies that currently have extensive and well- funded marketing and sales operations to recruit, hire, train and retain marketing and sales personnel. We also face competition in our search for third parties to assist us with the sales and marketing efforts of our product candidates. Without an internal team or the support of a third party to perform marketing and sales functions, we may be unable to compete successfully against these more established companies.
Physicians, patients, third-party payers or others in the medical community may not be receptive to our product candidates, and we may not generate any future revenue from the sale or licensing of our product candidates.
Even if we obtain approval for a product candidate, we may not generate or sustain revenue from sales of the product if the product cannot be sold at a competitive cost or if it fails to achieve market acceptance by physicians, patients, third-party payers or others in the medical community. These market participants may be hesitant to adopt a novel treatment based on ddRNAi or silence and replace technology, and we may not be able to convince the medical community and third-party payers to accept and use, or to provide favorable reimbursement for, any product candidates developed by us or our existing or future collaborators. Market acceptance of our product candidates will depend on, among other factors:
| • | the safety and efficacy of our product candidates; |
| • | our ability to offer our products for sale at competitive prices; |
| • | the relative convenience and ease of administration of our product candidates; |
| • | the prevalence and severity of any adverse side effects associated with our product candidates; |
| • | the terms of any approvals and the countries in which approvals are obtained; |
| • | limitations or warnings contained in any labeling approved by the FDA or comparable foreign regulatory authorities; |
| • | conditions upon the approval imposed by FDA or comparable foreign regulatory authorities, including, but not limited to, a REMS; |
| • | the willingness of patients to try new treatments and of physicians to prescribe these treatments; |
| • | the availability of government and other third-party payer coverage and adequate reimbursement; and |
| • | the availability of alternative effective treatments for the disease indications our product candidates are intended to treat and the relative risks, benefits and costs of those treatments. |
Since we are focused on the emerging therapeutic modality of ddRNAi, and silence and replace these risks may increase if new competitors are able to market ddRNAi-based therapeutics or silence and replace-based therapeutics or if these treatments become less favored in the commercial marketplace. In addition, we believe that one of the benefits of our ddRNAi and silence and replace technologies is the expected length of time of the effects. If our treatments do not have a long-term effect after administration, such a development would likely significantly and adversely affect market acceptance of our product candidates, if approved.
Additional risks apply in relation to any disease indications we pursue which are classified as rare diseases and allow for orphan drug designation by regulatory agencies in major commercial markets, such as the United States or European Union. If pricing is not approved or accepted in the market at an appropriate level for any approved product for which we pursue and receive an orphan drug designation, such product may not generate enough revenue to offset costs of development, manufacturing, marketing and commercialization despite any benefits
47
Table of Contents
received from the orphan drug designation, such as market exclusivity, for a period of time. Orphan exclusivity could temporarily delay or block approval of one of our products if a competitor obtains orphan drug designation for its product first. However, even if we obtain orphan exclusivity for one of our products upon approval, our exclusivity may not block the subsequent approval of a competitive product that is shown to be clinically superior to our product.
Market size is also a variable in disease indications not classified as rare. Our estimates regarding potential market size for any indication may be materially different from what we discover to exist at the time we commence commercialization, if any, for a product, which could result in significant changes in our business plan and have a material adverse effect on our business, financial condition, results of operations and prospects.
We face competition from entities that have developed or may develop product candidates for our target disease indications, including companies developing novel treatments and technology platforms based on modalities and technology similar to ours.
The development and commercialization of pharmaceutical products is highly competitive. We compete with a variety of multinational pharmaceutical companies and specialized biotechnology companies, as well as technology being developed at universities and other research institutions. Our competitors have developed, are developing or could develop product candidates and processes competitive with our product candidates. Competitive therapeutic treatments include those that have already been approved and accepted by the medical community, patients and third-party payers, and any new treatments that enter the market.
There may be a significant number of products that are currently under development, and may become commercially available in the future, for the treatment of conditions for which we are developing, and may in the future try to develop, product candidates. This increasingly competitive landscape may compromise the development of our product candidates.
We are aware of multiple companies that are working in the field of RNAi therapeutics, including Alnylam. Some of our current product candidates, if approved, would compete with approved and currently marketed treatments.
In addition, our ddRNAi-based product candidates would compete with antisense and other RNA-based pharmaceutical products currently under development. Like RNAi therapeutics, antisense products target mRNA with the objective of suppressing the activity of specific genes. The development of antisense products is more advanced than that of RNAi therapeutics, and antisense technology may become the preferred technology for products that target mRNAs.
Many of our competitors have significantly greater financial, technical, manufacturing, marketing, sales and supply resources and experience than we have. If we successfully obtain approval for any product candidate, we will face competition based on many different factors, including the safety and effectiveness of our products, the ease with which our products can be administered and the extent to which patients accept relatively new routes of administration, the timing and scope of regulatory approvals for these products, the availability and cost of manufacturing, marketing and sales capabilities, price, reimbursement coverage and patent position.
Competing products could present superior treatment alternatives, including by being more effective, safer, less expensive or marketed and sold more effectively than any products we may develop. Competitive products may make any products we develop obsolete or non-competitive before we recover the expense of developing and commercializing our product candidates. Such competitors could also recruit our employees, which could negatively impact our level of expertise and our ability to execute our business plan.
48
Table of Contents
A variety of risks outside of our control associated with international operations could adversely affect our business.
If any of our product candidates are approved for commercialization, it is our current intention to market them on a worldwide basis, either alone or in collaboration with others. In addition, we conduct development activities in various jurisdictions throughout the world. We expect that we will be subject to additional risks related to engaging in international operations, including:
| • | different regulatory requirements for approval of biopharmaceutical products in foreign countries; |
| • | reduced protection for intellectual property rights; |
| • | unexpected changes in tariffs, trade barriers and regulatory requirements; |
| • | economic weakness, including inflation, or political instability in particular foreign economies and markets; |
| • | compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; |
| • | foreign currency fluctuations, which could result in increased operating expenses and reduced revenue, and other obligations incident to doing business in another country; |
| • | workforce uncertainty in countries where labor unrest is more common than in Australia or the United States; |
| • | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and |
| • | business interruptions resulting from geopolitical actions, including war and terrorism, natural disasters, including earthquakes, typhoons, floods and fires and disease pandemics and epidemics. |
The insurance coverage and reimbursement status of newly approved products is uncertain.
The availability of coverage and adequate reimbursement by governmental and private payers is essential for most patients to be able to afford expensive treatments. Sales of our product candidates will depend substantially, both in the United States and abroad, on the extent to which the costs of our product candidates will be paid by health maintenance, managed care, pharmacy benefit and similar healthcare management organizations, or reimbursed by third-party payers, and there have been increasing efforts by governmental and other third-party payers, in the United States and abroad, to cap or reduce healthcare costs. If reimbursement is not available, or is available only to limited levels, we may not be able to successfully commercialize our product candidates. Even if coverage is provided, the reimbursement amounts approved by third-party payers may not be high enough to allow us to establish or maintain pricing sufficient to realize a return on our investment.
There is significant uncertainty related to the insurance coverage and reimbursement of newly approved products. In the United States, the principal decisions about reimbursement for new medicines are typically made by the Centers for Medicare & Medicaid Services, or CMS, an agency within the U.S. Department of Health and Human Services, as CMS decides whether and to what extent a new medicine will be covered and reimbursed under Medicare. Private payers tend to follow CMS to a substantial degree. It is difficult to predict what CMS will decide with respect to reimbursement for fundamentally novel products such as ours, as there is no body of established practices and precedents for these new products.
The intended use of a biopharmaceutical product by a physician can also affect pricing. For example, CMS could initiate a National Coverage Determination administrative procedure, by which the agency determines which uses of a therapeutic product would and would not be reimbursable under Medicare. This determination process can be lengthy, thereby creating a long period during which the future reimbursement for a particular product may be uncertain.
49
Table of Contents
Outside the United States, international operations are generally subject to extensive governmental price controls and other market regulations, and we believe the increasing emphasis on cost-containment initiatives in Europe, Canada and other countries is likely to put pressure on the pricing and usage of any of our product candidates that may be approved for marketing in the future. In many countries, the prices of medical products are subject to varying price control mechanisms as part of national health systems. In general, the prices of medicines under such systems can be substantially lower than in the United States. Other countries allow companies to fix their own prices for medicines, but monitor and control company profits. In some countries, we or our collaborators may be required to conduct a clinical trial or other studies that compare the cost-effectiveness of our product candidates to other available therapies in order to obtain or maintain reimbursement or pricing approval. Additional foreign price controls or other changes in pricing regulation could restrict the amount that we are able to charge for our product candidates. Accordingly, in markets outside the United States, the reimbursement for our products may be reduced compared with the United States and may be insufficient to generate commercially reasonable revenue and profits.
We expect to experience pricing pressures in connection with the sale of any of our product candidates due to the trend toward managed healthcare, value and risk-based payment arrangements that put healthcare providers at direct financial risk for the healthcare resources used to care for patient populations and additional legislative changes. The downward pressure on healthcare costs in general, particularly prescription pharmaceutical products and surgical procedures and other treatments, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products. We cannot predict what healthcare reform initiatives may be adopted in the future. Further federal, state and foreign legislative and regulatory developments are likely, and we expect ongoing initiatives to increase pressure on biopharmaceutical product pricing. Such reforms could depress pricing for any product candidates that we may successfully develop and for which we may obtain regulatory approval and may negatively affect our overall financial condition and ability to develop additional product candidates.
Changes in U.S. tax law may materially adversely affect our financial condition, results of operations and cash flows.
The rules dealing with U.S. federal, state, and local income taxation are constantly under review by persons involved in the legislative process and by the Internal Revenue Service and the U.S. Treasury Department. Changes to tax laws (which changes may have retroactive application) could adversely affect us or holders of our common stock, warrants and pre-funded warrants. In recent years, many such changes have been made and changes are likely to continue to occur in the future. Future changes in U.S. tax laws could have a material adverse effect on our business, cash flow, financial condition or results of operations. We urge our investors to consult with their legal and tax advisors regarding the implications of potential changes in U.S. tax laws on an investment in our common stock, warrants and pre-funded warrants.
Our relationships with third-party payers, healthcare professionals and customers in the United States and elsewhere may be subject to anti- kickback, fraud and abuse, false claims, transparency, health information privacy and security and other healthcare laws and regulations, which could expose us to significant penalties.
Our relationships with third-party payers, healthcare professionals and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations, including, without limitation, the federal Anti-Kickback Statute and the federal False Claims Act, that may constrain the business or financial arrangements and relationships through which we sell, market and distribute any biopharmaceutical products for which we obtain marketing approval. In addition, we may be subject to transparency laws and patient privacy regulation by the federal government and by the U.S. states and foreign jurisdictions in which we conduct our business. The applicable federal, state and foreign healthcare laws and regulations that may affect our ability to operate include, but are not limited to, the following:
| • | the federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, |
50
Table of Contents
| in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal and state healthcare programs such as Medicare and Medicaid; |
| • | federal civil and criminal false claims laws and civil monetary penalty laws, including the federal False Claims Act, which impose criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, including the Medicare and Medicaid programs, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| • | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which imposes criminal and civil liability for, among other things, executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
| • | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their respective implementing regulations, which impose obligations on covered healthcare providers, health plans and healthcare clearinghouses, as well as their business associates that create, receive, maintain or transmit individually identifiable health information for or on behalf of a covered entity, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| • | the federal Open Payments program, created under the ACA, and its implementing regulations, which requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to report annually to the CMS information related to payments or other transfers of value made to physicians, and applicable group purchasing organizations to report annually to CMS ownership and investment interests held by the physicians and their immediate family members by the 90th day of each subsequent calendar year, and disclosure of such information will be made by CMS on a publicly available website; and |
| • | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payers, including private insurers; state and foreign laws that require biopharmaceutical or biotechnology companies to comply with the industry voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers; state and foreign laws that require biopharmaceutical or biotechnology manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state and foreign laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations may involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, including, without limitation, damages, fines, disgorgement, imprisonment, exclusion from participation in government healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations, which could have a material adverse effect on our business. If any of the physicians or other healthcare providers or entities with whom we expect to do business is found not to be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from participation in government healthcare programs, which could also materially affect our business.
51
Table of Contents
Negative public opinion and increased regulatory scrutiny of gene therapy and genetic research may damage public perception of our product candidates or compromise our ability to conduct our business or obtain regulatory approvals for our product candidates.
Gene therapy remains a novel technology and no gene therapy product utilizing ddRNAi or silence and replace has been approved to date in the United States. Public perception may be influenced by claims that gene therapy is unsafe, and gene therapy may not gain the acceptance of the public or the medical community. In particular, our success will depend upon physicians specializing in the treatment of those diseases that our product candidates target prescribing treatments that involve the use of our product candidates in lieu of, or in addition to, existing treatments they are already familiar with and for which greater clinical data may be available. More restrictive government regulations or negative public opinion would have a negative effect on our business or financial condition and may delay or impair the development and commercialization of our product candidates or demand for any products we may develop. Our product candidates, including our viral delivery systems, could produce adverse events. Adverse events in our clinical trials or following approval of any of our product candidates, even if not ultimately attributable to our product candidates, could result in increased governmental regulation, unfavorable public perception, potential regulatory delays in the testing or approval of our product candidates, stricter labeling requirements for those product candidates that are approved and a decrease in demand for any such product candidates.
Risks Related to Our Business Operations
We may not successfully engage in strategic transactions or enter into new collaborations, which could adversely affect our ability to develop and commercialize product candidates, impact our cash position, increase our expenses and present significant distractions to our management.
From time to time, we may consider additional strategic transactions, such as collaborations, acquisitions, asset purchases or sales and out- or in- licensing of product candidates or technologies. In particular, we will evaluate and, if strategically attractive, seek to enter into additional collaborations, including with major biotechnology or pharmaceutical companies. The competition for collaborators is significant, and the negotiation process is time- consuming and complex. Anew collaboration may be on terms that are not optimal for us and we may not be to maintain any new or existing collaboration if, for example, development or approval of a product candidate is delayed, sales of an approved product candidate do not meet expectations or the collaborator discontinues the collaboration. Any such collaboration, or other strategic transaction, may require us to incur non- recurring or other charges, increase our expenditures, pose significant integration or implementation challenges or disrupt our management or business.
These transactions would entail numerous operational and financial risks, including exposure to unknown liabilities, incurrence of substantial debt or dilutive issuances of equity securities to pay transaction consideration or costs, higher than expected collaboration, acquisition or integration costs, write-downs of assets or goodwill or impairment charges, increased amortization expenses, difficulty and cost in facilitating the collaboration or combining the operations and personnel of any acquired business, impairment of relationships with key suppliers, manufacturers or customers of any acquired business due to changes in management and ownership and the inability to retain key employees of any acquired business.
Accordingly, although there can be no assurance that we will undertake or successfully complete any additional transactions of the nature described above, any transactions that we do complete may be subject to the foregoing or other risks and have a material adverse effect on our business, results of operations, financial condition and prospects. Conversely, any failure to enter any collaboration or other strategic transaction that would be beneficial to us could delay and make more expensive the development and potential commercialization of our product candidates and have a negative impact on the competitiveness of any product candidate that reaches market.
52
Table of Contents
Any inability to attract and retain qualified key management and technical personnel would impair our ability to implement our business plan.
Our success largely depends on the continued service of key management and other specialized personnel, including our chief executive officer, chief financial officer and chief operating officer. The loss of one or more members of our management team or other key employees or advisors, if not adequately replaced, could delay or increase the cost of our research and development programs and materially harm our business, financial condition, results of operations and prospects. The relationships that our key managers have cultivated within our industry make us particularly dependent upon their continued employment with us. We are dependent on the continued service of our technical personnel because of the highly technical nature of our product candidates and the specialized nature of the regulatory approval process for our product candidates. Because our management team and key employees are not obligated to provide us with continued service, they could terminate their employment with us at any time without penalty. We do not maintain key person life insurance policies on any of our management team members or key employees. Our future success will depend in large part on our continued ability to attract and retain other highly qualified scientific, technical and management personnel, as well as personnel with expertise in clinical testing, manufacturing, governmental regulation and commercialization. We face competition for personnel from other companies, universities, public and private research institutions, government entities and other organizations.
Our collaborations with outside scientists and consultants may be subject to restriction and change.
We work with medical experts, chemists, biologists and other scientists at academic and other institutions, and consultants who assist us in our research, development and regulatory efforts, including the members of our scientific advisory board. In addition, these scientists and consultants have provided, and we expect that they will continue to provide, valuable advice regarding our programs and regulatory approval processes. These scientists and consultants are not our employees and may have other commitments that would limit their future availability to us.
If a conflict of interest arises between their work for us and their work for another entity, we may lose their services. In addition, we are limited in our ability to prevent them from establishing competing businesses or developing competing products. For example, if a key scientist acting as a principal investigator in any of our future clinical trials identifies a potential product or compound that is more scientifically interesting to his or her professional interests, his or her availability to remain involved in clinical trials could be restricted or eliminated.
We may experience difficulties in managing our growth and expanding our operations.
We have limited experience in development and commercialization of pharmaceutical products. As our product candidates continue to advance through preclinical studies and any clinical trials and potentially toward regulatory approval and commercial sale, we will need to expand our development, regulatory, manufacturing and sales capabilities or contract with other organizations to provide these capabilities for us. In the future, we expect to have to manage additional relationships with collaborators or partners, suppliers and other organizations. Our ability to manage our operations and future growth will require us to continue to improve our operational, financial and management controls, reporting systems and procedures. We may not be able to implement improvements to our management information and control systems in an efficient or timely manner and may discover deficiencies in existing systems and controls.
Our employees, independent contractors, principal investigators, CROs, consultants and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and insider trading.
We are exposed to the risk of fraud or other misconduct by our employees, independent contractors, principal investigators, CROs, consultants, commercial partners and vendors. Misconduct by these parties could include
53
Table of Contents
intentional failures to comply with the regulations of the FDA and comparable foreign regulators, provide accurate information to the FDA and comparable foreign regulators, comply with healthcare fraud and abuse laws and regulations in the United States and abroad, report financial information or data accurately or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Such misconduct could also involve the improper use of information obtained in the course of any future clinical trials, which could result in regulatory sanctions and cause serious harm to our reputation. We have adopted a code of conduct, insider trading policy and other policies applicable to all of our employees, but it is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
We could face potential product liability and, if successful claims are brought against us, we may incur substantial liability and costs.
The use of our product candidates in clinical trials and the sale of any products for which we may in the future obtain marketing approval exposes us to the risk of product liability claims. Product liability claims might be brought against us by consumers, healthcare providers, pharmaceutical companies or others selling or otherwise coming into contact with our product candidates. There is a risk that our product candidates may induce adverse events. If we cannot successfully defend against product liability claims, we could incur substantial liability and costs. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| • | impairment of our business reputation; |
| • | withdrawal of clinical trial participants; |
| • | costs due to related litigation; |
| • | distraction of management’s attention from our primary business; |
| • | substantial monetary awards to patients or other claimants; |
| • | the inability to commercialize our product candidates; |
| • | decreased demand for our product candidates, if approved for commercial sale; and |
| • | increased cost, or impairment of our ability, to obtain or maintain product liability insurance coverage. |
We carry customary levels of general public and products liability (including human clinical trials extension) insurance We believe our product liability insurance coverage is sufficient in light of our current clinical programs. However, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. If we obtain marketing approval for any product candidates, we intend to expand our insurance coverage to include the sale of commercial products, but we may not be able to obtain or maintain product liability insurance on commercially reasonable terms or in adequate amounts. On occasion, large judgments have been awarded against other pharmaceutical companies in class action lawsuits based on pharmaceutical products, or medical treatments that had unanticipated adverse effects. A successful product liability claim or series of claims brought against us could cause the price of our common stock to
54
Table of Contents
decline and, if judgments exceed our insurance coverage, could materially and adversely affect our financial position. During the course of treatment, patients may suffer adverse events, including death, for reasons that may be related to our product candidates. Such events could subject us to costly litigation, require us to pay substantial amounts of money to injured patients, delay, negatively impact or end our opportunity to receive or maintain regulatory approval to market our products, or require us to suspend or discontinue our commercialization efforts. Even in a circumstance in which we do not believe that an adverse event is related to our product candidate, the investigation into the circumstance may be time-consuming or inconclusive. These investigations may harm our reputation, delay our regulatory approval process, limit the type of regulatory approvals our product candidates receive or maintain, and compromise the market acceptance of any of our product candidates that may in the future receive regulatory approval. As a result of these factors, a product liability claim, even if successfully defended, could hurt our business and impair our ability to generate revenue.
We and our development partners, third-party manufacturers and suppliers use biological materials and may use hazardous materials, and any claims relating to improper handling, storage or disposal of these materials could be time consuming or costly.
We and our development partners, third-party manufacturers and suppliers may use hazardous materials, including chemicals and biological agents and compounds that could be dangerous to human health and safety or the environment. Our operations and the operations of our third-party manufacturers and suppliers also produce hazardous waste products. National, state and local laws and regulations in the United States and other countries govern the use, generation, manufacture, storage, handling and disposal of these materials and wastes. Compliance with applicable environmental laws and regulations may be expensive, and current or future environmental laws and regulations may impair our product development and commercialization efforts. In addition, we cannot entirely eliminate the risk of accidental injury or contamination from these materials or wastes. We do not carry specific biological or hazardous waste insurance coverage, and our property, casualty and general liability insurance policies specifically exclude coverage for damages and fines arising from biological or hazardous waste exposure or contamination. Accordingly, in the event of contamination or injury, we could be held liable for damages or be penalized with fines in an amount exceeding our resources, and any future clinical trials, regulatory approvals or product commercialization progress could be suspended.
We may use our limited financial and human resources to pursue a particular research program or product candidate and fail to capitalize on programs or product candidates that may be more profitable or for which there is a greater likelihood of success.
Because we have limited resources, we may forego or delay pursuit of opportunities with certain programs or product candidates or for indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs for product candidates may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through strategic collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate, or we may allocate internal resources to a product candidate in a therapeutic area in which it would have been more advantageous to enter into a collaboration arrangement.
Our internal computer and information technology systems, or those of our collaborators and other development partners, third-party CROs or other contractors or consultants, may fail or suffer security breaches, which could result in a disruption of our product development programs.
Despite the implementation of security measures, our internal computer and information technology systems and those of our current and any future CROs and other contractors, consultants and collaborators are vulnerable to damage from computer viruses, cyber-attacks, unauthorized access, natural disasters, terrorism, war and
55
Table of Contents
telecommunication and electrical failures. Such events could cause interruptions of our operations. While we have not experienced any material system failure, accident or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a disruption of our development programs and our business operations, whether due to a loss of our trade secrets or other similar disruptions. For example, the loss of clinical trial data from ongoing or future clinical trials or data from preclinical studies could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Likewise, we rely on third parties to manufacture our product candidates and will rely on third parties to conduct future clinical trials, and similar events relating to their computer systems could also have similar consequences to our business. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development and commercialization of our product candidates could be delayed and become more expensive. We may face increased cybersecurity risks due to our reliance on internet technology and the number of our employees that are working remotely, which may create additional opportunities for cybercriminals to exploit vulnerabilities.
Cyber-attacks, breaches, interruptions or other data security incidents could result in legal claims or proceedings, liability under federal or state laws that protect the privacy of personal information, regulatory penalties, significant remediation costs, disrupt key business operations and divert attention of management and key information technology resources. In the United States, notice of breaches must be made to affected individuals, the U.S. Secretary of the Department of Health and Human Services, or HHS, and for extensive breaches, notice may need to be made to the media or U.S. state attorneys general. Such a notice could harm our reputation and our ability to compete. HHS has the discretion to impose penalties without attempting to resolve violations through informal means. In addition, U.S. state attorneys general are authorized to bring civil actions seeking either injunctions or damages in response to violations that threaten the privacy of state residents. There can be no assurance that we, our collaborators, CROs, vendors, and any other business counterparties will be successful in efforts to detect, prevent, protect against or fully recover systems or data from all breakdowns, service interruptions, attacks or breaches of systems. In addition, we do not maintain standalone cyber-security insurance and have limited insurance coverage in the event of any breach or disruption of our or our collaborators’, CROs’, or vendors’ systems, including any unauthorized access or loss of any personal data that we may collect, store or otherwise process. The costs related to significant security breaches or disruptions could be material and exceed the limits of any insurance coverage we may have. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or systems, or inappropriate disclosure of confidential or proprietary information, including data related to our personnel, we could incur liability and the further development and commercialization of our product candidates could be delayed and our business and operations could be adversely affected and/or could result in the loss or disclosure of critical or sensitive data, which could result in financial, legal, business or reputational harm to us.
Our current laboratory operations are concentrated in one location and any events affecting this location may seriously compromise our ability to operate our business and continue the development of our product candidates.
Our current laboratory operations are located in our facility situated in Hayward, California. Any unplanned event, such as flood, fire, explosion, earthquake, extreme weather condition, medical epidemics and pandemics, power shortage, telecommunication failure or other natural or manmade accidents or incidents that result in us being unable to fully utilize the facility, may compromise our ability to operate our business, particularly on a daily basis, cause us financial losses and inhibit or delay our continued development of our product candidates. Loss of access to this facility may result in increased costs, delays in the development of our product candidates or interruption of our business operations. As part of our risk management policy, we maintain insurance coverage at levels that we believe are appropriate for our business. However, in the event of an accident or incident at this facility, we cannot assure you that the amounts of insurance will be sufficient to satisfy any damages and losses. If our facility is unable to operate because of an accident or incident or for any other reason, even for a short period of time, any or all of our research and development programs may be harmed. Any
56
Table of Contents
business interruption may have a material adverse effect on our business, financial position, results of operations and prospects.
The investment of our cash and cash equivalents is subject to risks which may cause losses and affect the liquidity of these investments.
As of June 30, 2025, we had $97.7 million in cash and cash equivalents. We historically have invested substantially all of our available cash and cash equivalents in cash deposits meeting the criteria of our investment policy, which is focused on the preservation of our capital. These investments are subject to general credit, liquidity, market and interest rate risks. We may realize losses in the fair value of these investments, which would have a negative effect on our financial results. In addition, should our investments cease paying or reduce the amount of interest paid to us, our interest income would suffer. The market risks associated with our investment portfolio may have an adverse effect on our results of operations, liquidity and financial condition.
Risks Related to Our Intellectual Property
If we are unable to obtain or protect sufficient intellectual property rights related to our product candidates, we may not be able to obtain exclusivity for our product candidates or prevent others from developing similar competitive products.
We rely upon a combination of patents, know-how, trade secret protection and confidentiality agreements—both that we own or possess or that are owned or controlled by our licensors and licensed to us—to protect the intellectual property related to our technology and product candidates.
The patent application process, also known as patent prosecution, is expensive and time-consuming, and we and our current or future licensors and licensees may not be able to prepare, file, and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we or our current licensors or licensees, or any future licensors or licensees, will fail to identify or obtain sufficient protection for patentable aspects of inventions made in the course of development and commercialization activities before it is too late to obtain patent protection on them. Therefore, our patents and patent applications may not be prosecuted and enforced in a manner consistent with the best interests of our business. It is possible that defects of form in the preparation or filing of our patents or patent applications may exist, or may arise in the future, for example with respect to proper priority claims, inventorship, claim scope, patent term adjustments, etc., although we are unaware of any such defects. If we or our current licensors or licensees, or any future licensors or licensees, fail to establish, maintain or protect such patents and other intellectual property rights, such rights may be reduced or eliminated. If our current licensors or licensees, or any future licensors or licensees, are not fully cooperative or disagree with us as to the prosecution, maintenance or enforcement of any patent rights, such patent rights could be compromised. If there are material defects in the form or preparation of our patents or patent applications, such patents or applications may be invalid and unenforceable in circumstances where it is not possible to remedy those material defects. Any of these outcomes could impair our ability to prevent competition from third parties, which may have an adverse impact on our business.
The strength of patents in the biotechnology and pharmaceutical field involves complex legal and scientific questions and can be uncertain. The patent applications that we own or in-license may fail to result in issued patents with claims that cover our product candidates in the United States or other jurisdictions. In addition, we cannot guarantee that any patents will issue from any pending or future patent applications owned by or licensed to us. There is no assurance that all of the potentially relevant prior art relating to our patents and patent applications has been found. If such prior art exists, it can invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue and even if such patents cover our product candidates, third parties may initiate opposition, interference, re-examination, post-grant review, inter parties review, nullification or derivation action in court or before patent offices or similar proceedings challenging the validity, enforceability or scope of such patents, which may result in the patent claims being
57
Table of Contents
narrowed or invalidated. Furthermore, even if our patents and patent applications are unchallenged, they may not adequately protect our intellectual property, provide exclusivity for our product candidates or prevent others from designing around our claims. Any of these outcomes could impair our ability to prevent competition from third parties.
If the patent applications we hold or have in-licensed with respect to our programs or product candidates fail to issue, or are revoked, if the breadth or strength of our patent protection is threatened, or if our patent portfolio fails to provide meaningful exclusivity for our product candidates, it could dissuade companies from collaborating with us to develop product candidates and threaten our ability to commercialize future products. Any successful opposition to any patents owned by or licensed to us could deprive us of rights necessary for the successful commercialization of any product candidates that we may develop. Further, if we encounter delays in regulatory approvals, the period of time during which we could market a product candidate under patent protection could be reduced. Since patent applications in the United States and most other countries are confidential for a period of time (up to 18 months) after filing, we cannot be certain that we or our licensors were the first to file any patent application related to a product candidate. Furthermore, if third parties have filed such patent applications before March 16, 2013, an interference proceeding in the United States can be initiated by such third parties to determine who was the first to invent any of the subject matter covered by the patent claims of our applications. If third parties have filed such applications on or after March 16, 2013, a derivation proceeding in the United States can be initiated by such third parties to determine whether our invention was derived from theirs. Even where we have a valid and enforceable patent, we may not be able to exclude others from practicing our invention where the other party can show that they used the invention in commerce before our filing date or the other party benefits from a compulsory license. In addition, patents have a limited lifespan. In the United States, and in most other jurisdictions with a patent system, the natural expiration of a patent is generally 20 years after its filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our product candidates are obtained, once the patent life has expired for a product, we may be open to competition from competitive medications, including biosimilar or generic medications. This risk is material in light of the length of the development process of our products and lifespan of our current patent portfolio.
In addition to the protection afforded by patents, we rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or that we elect not to patent, processes for which patents are difficult to enforce and any other elements of our product candidate discovery and development processes that involve proprietary know-how, information or technology that is not covered by patents. However, trade secrets can be difficult to protect. What constitutes a trade secret and what protections are available for trade secrets varies from state to state in the United States and country by country worldwide. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with our employees, consultants, scientific advisors and contractors. We also seek to preserve the integrity and confidentiality of our data and trade secrets by measures designed to maintain the physical security of our premises and physical and electronic security of our information technology systems. Security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known to or be independently discovered by competitors. Although we expect all of our employees and consultants to assign their inventions to us, and all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information or technology to enter into confidentiality agreements, we cannot provide any assurances that all such agreements have been duly executed, that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Misappropriation or unauthorized disclosure of our trade secrets can be difficult to detect, could impair our competitive position and may have a material adverse effect on our business. Additionally, if the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third parties for misappropriating the trade secret. In addition, others may independently discover our trade secrets and proprietary information. For example, the FDA, as part of its Transparency Initiative, is currently considering whether to make additional information publicly available on a routine basis, including information that we may
58
Table of Contents
consider to be trade secrets or other proprietary information, and it is not clear at the present time how the FDA’s disclosure policies may change in the future, if at all.
Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. Further, the laws of some foreign countries such as India and China do not protect proprietary rights to the same extent or in the same manner as the laws of the United States. As a result, we may encounter significant problems in protecting and defending our intellectual property both in the United States and abroad. If we are unable to prevent material disclosure of the non-patented intellectual property related to our technologies to third parties, and there is no guarantee that we will have any such enforceable trade secret protection, we may not be able to establish or maintain a competitive advantage in our markets.
We rely on license relationships with a number of third parties for portions of our intellectual property, including platform technology patents relating to our ddRNAi technology.
We have in-licensed certain intellectual property from third parties, including technology related to ddRNAi and antisense RNA. In some cases, our licenses to intellectual property are non-exclusive and the licensors may license the technology to our competitors in the same field, which may result is significant competition for us. In other cases, our licenses to intellectual property are exclusive only for a specific field of use (such as human therapeutics), and the licensors retain rights to practice the licensed intellectual property themselves and to grant licenses to third parties in other fields. Such third parties may develop improvements to the licensed intellectual property that are not licensed to us, which could block our ability to continue developing the product candidate covered by the licensed intellectual property, unless we negotiate a license. Such third parties may also disclose competitively sensitive information about the licensed intellectual property that diminishes its value. In other cases, our licenses are for research purposes only. Upon regulatory marketing approval of our product candidates it may be necessary for us to obtain a broader license in order to commercialize. We cannot guarantee the availability of the broader license or that it can be obtained on commercially reasonable terms.
We rely on some of these third party licensors to file and prosecute patent applications and maintain patents and otherwise protect the intellectual property we license. We have not had and do not have primary control over these activities for certain of our patents or patent applications and other intellectual property rights we license, and therefore cannot guarantee that these patents and applications will be prosecuted or enforced in a manner consistent with the best interests of our business. We cannot be certain that such activities by third parties have been or will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents and other intellectual property rights. Additionally, we may not be able to control the publication or other disclosures of research carried out by our licensors relating to technology that could otherwise prove patentable. Pursuant to the terms of some of our license agreements with third parties, some of our third-party licensors have the right, but not the obligation, to control enforcement of our licensed patents or defense of any claims asserting the invalidity of these patents. Even if we are permitted to pursue such enforcement or defense, we will require the cooperation of our licensors, and cannot guarantee that we would receive it and on what terms. We cannot be certain that our licensors will allocate sufficient resources or prioritize their or our prosecution or enforcement of such patents or defense of such claims to protect our interests in the licensed patents. If we cannot obtain patent protection, or enforce existing or future patents against third parties, our competitive position and our financial condition could suffer.
If we fail to comply with our obligations in the agreements under which we license intellectual property rights from third parties or otherwise experience disruptions to our business relationships with our licensors, we could lose license rights that are important to our business.
We hold rights under license or sublicense agreements with third parties that are important to our business. Under our existing license and sublicense agreements, we are subject to various obligations, including diligence obligations with respect to development and commercialization activities and payment obligations. In spite of our
59
Table of Contents
efforts, our licensors may conclude that we have materially breached our obligations under such license agreements and terminate the license agreements, thereby removing or limiting our ability to develop and commercialize product candidates and technology covered by these license agreements.
We may need to obtain additional licenses from third parties to advance our research or allow commercialization of our product candidates, such as if we identify new technology that would advance our programs or if an existing license agreement is terminated. We may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. In that event, we may be required to expend significant time and resources to develop or license replacement technology. If we are unable to do so, we may be unable to develop or commercialize the affected product candidates.
In many cases, patent prosecution of our in-licensed technology is controlled solely by the licensor. If our licensors fail to obtain and maintain patent or other protection for the proprietary intellectual property we license from them, we could lose our rights to the intellectual property or our exclusivity with respect to those rights, and our competitors could market competing products using the intellectual property. In some cases, we control the prosecution of patents resulting from licensed technology. In the event we breach any of our obligations related to such prosecution, we may incur significant liability to our licensing partners and the value of the licensed patents may be adversely affected.
Licensing of intellectual property is of critical importance to our business and involves complex legal, business and scientific issues and is complicated by the rapid pace of scientific discovery in our industry. Disputes may arise regarding intellectual property subject to a licensing agreement, including:
| • | the scope of rights granted under the license agreement and other interpretation-related issues; |
| • | the extent to which our technology and processes infringe on intellectual property of a licensor that is not subject to the licensing agreement; |
| • | the sublicensing of patent and other rights under any collaborative relationships we might enter into in the future; |
| • | our diligence obligations under the license agreement and what activities satisfy those diligence obligations; |
| • | the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and |
| • | the priority of invention of patented technology. |
If disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates.
Our results of operations will be affected by the level of royalty payments that we are required to pay to third parties.
We have been and may in the future be party to license agreements that require us to remit royalty payments and other payments related to in-licensed intellectual property. Under in-license agreements, we may be required to pay up-front fees and milestone payments and be subject to future royalties. We cannot precisely predict the amount, if any, of royalties we will owe in the future, and if our calculations of royalty payments are incorrect, we may owe additional royalties, which could negatively affect our results of operations. As our product sales increase, we may, from time to time, disagree with our third-party collaborators as to the appropriate royalties owed, and the resolution of such disputes may be costly, may consume management’s time, and may damage our relationship with our collaborators. Furthermore, we may enter into additional license agreements in the future, which may also include royalty, milestone and other payments.
60
Table of Contents
The licenses we may grant to our collaborators and other licensees to use our ddRNAi and other technology may be exclusive to the development of product candidates for certain conditions.
Some of the out-licenses we may grant to our collaborators to use our ddRNAi and other technology may be exclusive to the development of product candidates for certain conditions, so long as our collaborators comply with certain requirements. That means that once our ddRNAi technology is licensed to a collaborator for a specified condition, we are generally prohibited from developing product candidates for that condition and from licensing the ddRNAi to any third party for that condition. The limitations imposed by these exclusive licenses could prevent us from expanding our business and increasing our development of product candidates with new collaborators, both of which could adversely affect our business and results of operations.
Third-party claims of intellectual property infringement may prevent or delay our development and commercialization efforts.
Our commercial success depends in part on our avoiding infringement of the patents and proprietary rights of third parties. There is a substantial amount of litigation, both within and outside the United States, involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries, including patent infringement lawsuits, interferences, oppositions and inter parties review proceedings before the USPTO, and corresponding foreign patent offices. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are pursuing development candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our product candidates may be subject to claims of infringement of the patent rights of third parties.
Third parties may assert that we are employing their proprietary technology without authorization. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of our product candidates. Because patent applications can take many years to issue, there may be currently pending patent applications which may later result in issued patents that our product candidates may be accused of infringing. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. If any third-party patents were held by a court of competent jurisdiction to cover the manufacturing process, methods of use or formulations of any of our product candidates, any DNA constructs formed during the manufacturing process or any final product itself, the holders of any such patents may be able to block our ability to commercialize such product candidate unless we obtained a license under the applicable patents, or until such patents expire. In either case, such a license may not be available on commercially reasonable terms or at all. Parties making claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize one or more of our product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of management and employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, pay royalties, redesign our infringing products or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure. Even if a license can be obtained on acceptable terms, the rights may be non-exclusive, which could give our competitors access to the same technology or intellectual property rights licensed to us. If we fail to obtain a required license, we may be unable to effectively market product candidates based on our technology, which could limit our ability to generate revenue or achieve profitability and possibly prevent us from generating revenue sufficient to sustain our operations.
We may not be successful in obtaining or maintaining necessary rights to gene therapy product components and processes for our development pipeline through acquisitions and in-licenses.
Presently, we have certain rights to intellectual property to develop our current gene therapy product candidates. However, our product candidates may require specific formulations to work effectively and efficiently and rights to such formulations may be held by others. In addition, we may need additional intellectual property rights as
61
Table of Contents
we develop future product candidates. In particular, we are aware of a third party patent directed to AAV vectors that expires in 2026. In the event we receive regulatory marketing approval before the expiration date it may be necessary for us to obtain a license to the patent in order to commercialize. We cannot guarantee the availability of the license or that it can be obtained on commercially reasonable terms.
We may be unable to acquire or in-license any compositions, methods of use, processes or other third-party intellectual property rights from third parties that we identify on terms that we find acceptable, or at all. The licensing and acquisition of third-party intellectual property rights is a competitive area, and a number of more established companies are also pursuing strategies to license or acquire third-party intellectual property rights that we may consider attractive. These established companies may have a competitive advantage over us due to their size, cash resources and greater clinical development and commercialization capabilities.
For example, we sometimes collaborate with U.S. and foreign academic institutions to accelerate our preclinical research or development under written agreements with these institutions. Typically, these institutions provide us with an option to negotiate a license to any of the institution’s rights in technology resulting from the collaboration. Regardless of such right of first negotiation for intellectual property, we may be unable to negotiate a license within the specified time frame or under terms that are acceptable to us. If we are unable to do so, the institution may offer the intellectual property rights to other parties, potentially blocking our ability to pursue our program.
In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on our investment. If we are unable to successfully obtain rights to required third- party intellectual property rights, our business, financial condition and prospects for growth could suffer.
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming and unsuccessful, and issued patents covering our product candidates could be found invalid or unenforceable if challenged in court.
Competitors may infringe our patents or the patents of our licensors. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours or our licensors is not valid, is unenforceable or is not infringed, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing.
For example, in patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, for example, lack of novelty, obviousness or non- enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. The outcome following legal assertions of invalidity and unenforceability during patent litigation is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re-examination, post grant review, and equivalent proceedings in foreign jurisdictions, such as opposition proceedings. Such proceedings could result in revocation, amendments to our patent claims or statements being made on the record such that our claims may no longer be construed to cover our product candidates. Outcomes or statements on the record in one country could have a disadvantageous effect on prosecution or enforcement of a patent or patent application in another country. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that no
62
Table of Contents
invalidating prior art exists or that the patent examiner was aware of all material prior art during prosecution. Moreover, in some circumstances, we do not have the right to control the preparation, filing and prosecution of patent applications, or to maintain, enforce or defend the patents, covering technology that we license from third parties. Therefore, these patents and applications may not be prosecuted, enforced and defended in a manner consistent with the best interests of our business. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we could lose at least part, and perhaps all, of the patent protection on one or more of our products or certain aspects of our platform technology. Such a loss of patent protection could have a material adverse impact on our business. Patents and other intellectual property rights also will not protect our technology if competitors design around our protected technology without legally infringing our patents or other intellectual property rights. Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses and could distract our technical and management personnel from their normal responsibilities. In addition, enforcement of a favorable decision by a court can depend on cooperation of a governmental authority which may or may not be available in every jurisdiction. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities.
For our patents and patent applications filed in the United States before March 16, 2013, interference proceedings provoked by third parties or brought by us may be necessary to determine the priority of inventions with respect to our patents or patent applications or those of our licensors. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Litigation or interference proceedings may result in a decision adverse to our interests and, even if successful, may result in substantial costs and distract our management and other employees. We may not be able to prevent, alone or with our licensors, misappropriation of our intellectual property rights, particularly in countries where the laws may not protect those rights as fully as in the United States.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could cause the trading price of our common stock to fall.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Certain of our key employees and personnel are or were previously employed at universities, medical institutions or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants and independent contractors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of any of our employee’s former employer or other third parties. Litigation may be necessary to defend against these claims. Furthermore, universities or medical institutions who employ some of our key employees and personnel in parallel to their engagement by us may claim that intellectual property developed by such person is owned by the respective academic or medical institution under the respective institution intellectual property policy or applicable law. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs, be a distraction to management and other employees, and damage our relationships with the academic and medical institutions.
63
Table of Contents
We may be subject to claims challenging the inventorship or ownership of our patents and other intellectual property.
We may be subject to claims that former employees, collaborators or other third parties have an ownership interest in our patents or other intellectual property. We may in the future have ownership disputes arising, for example, from conflicting obligations of consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship or ownership. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and applications are required to be paid to the USPTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents and applications. The USPTO and various corresponding governmental patent agencies outside of the United States require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process and after a patent has issued. There are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
As is the case with other biotechnology companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biotechnology industry involve both technological and legal complexity, and it therefore is costly, time-consuming and inherently uncertain. In addition, on September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law, including provisions that affect the way patent applications will be prosecuted and may also affect patent litigation.
An important change introduced by the Leahy-Smith Act is that, as of March 16, 2013, the United States transitioned to a “first-to-file” system for deciding which party should be granted a patent when two or more patent applications are filed by different parties claiming the same invention. A third party that files a patent application in the USPTO after that date but before us could therefore be awarded a patent covering an invention of ours even if we had made the invention before it was made by the third party. This will require us to be cognizant going forward of the time from invention to filing of a patent application. It is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
Among some of the other changes introduced by the Leahy-Smith Act are changes that limit where a patentee may file a patent infringement suit and providing opportunities for third parties to challenge any issued patent in the USPTO. This applies to all of our U.S. patents, even those issued before March 16, 2013. Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in U.S. federal court necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action.
64
Table of Contents
Recent U.S. Supreme Court rulings such as Association for Molecular Pathology v. Myriad Genetics, Inc. (Myriad I); BRCA1- & BRCA2-Based Hereditary Cancer Test Patent Litig. (Myriad II); and Promega Corp. v. Life Technologies Corp. have also narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in some situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
Our success depends, in part, on our ability to protect our intellectual property and our technologies outside the United States.
Our commercial success depends, in part, on our ability to obtain and maintain patent and trade secret protection for our technologies, our traits, and their uses, as well as our ability to operate without infringing upon the proprietary rights of others outside the United States. If we do not adequately protect our intellectual property, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our business and ability to achieve profitability.
Filing, prosecuting and defending patents on product candidates in all countries around the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. In addition, we may at times in- license third-party technologies for which limited international patent protection exists and for which the time period for filing international patent applications has passed. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Potential competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our product candidates, if approved, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Risks Related to an Investment in Our Common Stock
A number of our stockholders hold significant amounts of our common stock and unexercised warrants to acquire common stock, and therefore could exert significant influence over us.
While our stockholder base and relative holdings may change over time, a number of institutional investors and similar stockholders currently hold significant ownership positions in our outstanding common stock and outstanding Warrants to acquire Common Stock.
65
Table of Contents
On September 26, 2024, Suvretta Capital, on behalf of itself and each of the Suvretta Funds, entered into a waiver with the Company, pursuant to which, among other things (i) Suvretta Capital waived the 19.99% beneficial ownership limitation set forth in each of the warrants held by the Suvretta Funds, and (ii) Suvretta Capital and the Company agreed that Suvretta Capital will not be permitted to complete an exercise of the warrants held by the Suvretta Funds to the extent the beneficial ownership (calculated as provided in the applicable warrants) of Suvretta Capital in the Company following such exercise would exceed 49.9%. In addition, in connection with the April 2024 private placement, we entered into the Board Designation Agreement with Suvretta Capital pursuant to which the Company appointed Kishen Mehta to the Board.
The interests of these significant stockholders might not always coincide with the interests of other stockholders, and any influence exerted over our business and affairs by these significant stockholders directly or through an appointee to the Board might not always coincide with the wishes of other stockholders. In addition, the control and influence held by these significant stockholders might have the effect of delaying, deferring, or preventing a transaction or change in control of us, which might involve a premium price for shares of our Common Stock, or which otherwise could have been in your best interests as a stockholder.
The market price and trading volume of our common stock may be volatile and may be affected by economic conditions beyond our control.
The market price of our common stock may be highly volatile and subject to wide fluctuations. In addition, the trading volume of our common stock may fluctuate and cause significant price variations to occur. If the market price of our common stock declines significantly, you may be unable to resell your shares of our common stock at or above your purchase price, if at all. We cannot assure you that the market price of our common stock will not fluctuate or significantly decline in the future.
Some specific factors that could negatively affect the price of our common stock or result in fluctuations in its price and trading volume include:
| • | results of our clinical trials; |
| • | regulatory actions; |
| • | actual or expected fluctuations in our operating results; |
| • | changes in market valuations of similar companies; |
| • | changes in our key personnel; |
| • | changes in financial estimates or recommendations by securities analysts; |
| • | strategic decisions by us or our competitors, such as acquisitions, collaborations, divestitures, spin-offs, joint ventures, strategic investments or changes in business strategy; |
| • | the passage of legislation or other regulatory developments in the United States and other countries affecting us or our industry; |
| • | changes in trading volume of our common stock on Nasdaq; |
| • | sales of our common stock by us, our executive officers or our stockholders in the future; and |
| • | conditions in the financial markets or changes in general economic conditions. |
In addition, the stock market has experienced and is currently experiencing significant volatility, particularly with respect to pharmaceutical, biotechnology and other life sciences company stocks. The volatility of pharmaceutical, biotechnology and other life sciences company stocks often does not relate to the operating performance of the companies represented by the stock.
66
Table of Contents
An active trading market for our common stock may not continue to develop or may not be liquid enough for you to sell your shares of our common stock quickly or at market price.
Although our common stock is listed on Nasdaq, if an active public market in the United States for our common stock does not continue to develop, the market price and liquidity of our common stock may be adversely affected. The price of our common stock may decline, which means you may experience a decrease in the value of your shares of our common stock regardless of our operating performance or prospects. In the past, following periods of volatility in the market price of a company’s securities, stockholders often instituted securities class action litigation against that company. If we were involved in a class action suit, it could divert the attention of senior management and, if adversely determined, could cause us significant financial harm.
If securities or industry analysts do not continue to publish research or reports, or if they publish adverse or misleading research or reports, regarding us, our business or our market, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that securities or industry analysts publish about us, our business or our market. If no additional securities or industry analysts commence coverage of us, our stock price could be negatively impacted. If any of the analysts who cover us issue adverse or misleading research or reports regarding us, our business model, our intellectual property, our stock performance or our market, or if our operating results fail to meet the expectations of analysts, our stock price would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
If we fail to establish and maintain proper internal financial reporting controls, our ability to produce accurate financial statements or comply with applicable regulations could be impaired.
Section 404(a) of the Sarbanes-Oxley Act requires that our management assess and report annually on the effectiveness of our internal controls over financial reporting and identify any material weaknesses in our internal controls over financial reporting. Although Section 404(b) of the Sarbanes- Oxley Act requires our independent registered public accounting firm to issue an annual report that addresses the effectiveness of our internal controls over financial reporting, we rely on the exemption provided for non-accelerated filers, and consequently will not be required to comply with SEC rules that implement Section 404(b) of the Sarbanes-Oxley Act until such time as we are an accelerated or large accelerated filer.
The presence of any material weaknesses in our internal control over financial reporting could result in financial statement errors which, in turn, could lead to errors in our financial reports and/or delays in our financial reporting, which could require us to restate our operating results. We might not identify one or more material weaknesses in our internal controls in connection with evaluating our compliance with Section 404(a) of the Sarbanes- Oxley Act. In order to maintain and improve the effectiveness of our disclosure controls and procedures and internal controls over financial reporting, we will need to expend significant resources and provide significant management oversight. Implementing any appropriate changes to our internal controls may require specific compliance training of our directors and employees, entail substantial costs in order to modify our existing accounting systems, take a significant period of time to complete and divert management’s attention from other business concerns. These changes may not, however, be effective in maintaining the adequacy of our internal control.
The report of our management for the fiscal year ended June 30, 2025 is included in Item 9A – Controls and Procedures of this Annual Report on Form 10-K. As further outlined in that report, our management has concluded that our internal control over financial reporting was not effective as of June 30, 2025, due to a material weakness in our internal controls in connection with inadequate design and implementation of controls over our share-based compensation calculation review process. This material weakness resulted in the restatement of our unaudited condensed consolidated financial statements as of and for the quarterly periods
67
Table of Contents
ended March 31, 2025, and December 31, 2024, included in this Annual Report on Form 10-K. Refer to Note 3, Restatement of Prior Period Financial Statements, in the consolidated financial statements in Part II, Item 8 for additional information.
Any failure to remediate the identified material weakness, or to develop or maintain effective controls, or any difficulties encountered in the implementation or improvement of such controls, could harm our operating results or cause us to fail to meet our reporting obligations and may result in a restatement of our financial statements for prior periods, such as the restatement of our previously issued unaudited condensed consolidated financial statements described in more detail in this Annual Report on Form 10-K. Any failure to remediate the identified material weakness, or to implement and maintain effective internal control over financial reporting also could adversely affect the results of management evaluations and independent registered public accounting firm audits of our internal control over financial reporting that we are required to include in our periodic reports that will be filed with the SEC. We can provide no assurance that the measures we are taking and plan to take in the future will remediate the material weakness identified in connection with the restatement, or that any additional material weaknesses or restatements of financial results will not arise in the future due to a failure to implement and maintain adequate internal control over financial reporting or circumvention of these controls. In addition, even if we are successful in strengthening our controls and procedures, in the future those controls and procedures may not be adequate to prevent or identify irregularities or errors or to facilitate the fair presentation of our financial statements. We continue to evaluate steps to remediate the material weakness.
Such remedial measures will take time to implement and test and there can be no assurance that such measures will be sufficient to remedy the material weakness around stock-based compensation identified or that additional material weaknesses or other control or significant deficiencies will not be identified in the future. If we continue to experience material weaknesses in our internal controls or fail to maintain or implement required new or improved controls, investors may lose confidence in our operating results, the price of our common stock could decline and we may be subject to litigation or regulatory enforcement actions. In addition, if we are unable to meet the requirements of Section 404 of the Sarbanes-Oxley Act, our common stock may not be able to remain listed on Nasdaq.
We have restated certain of our prior issued interim financial statements, which has resulted in unanticipated costs and may lead to additional risks and uncertainties, including loss of investor confidence and, as a result, the value of our common stock.
As discussed in Note 3 to our consolidated financial statements included elsewhere in this Annual Report on Form 10-K, we determined to restate our previously issued unaudited condensed consolidated financial statements as of and for the quarterly periods ended March 31, 2025, and December 31, 2024, after we identified the inaccurate calculation of share-based compensation expense due to a system configuration issue that was not detected by our share-based compensation review controls. As a result of this error and the resulting restatement of our unaudited condensed consolidated financial statements for the impacted periods, we have incurred, and may continue to incur, unanticipated costs for accounting and legal fees in connection with or related to the restatement and have become subject to a number of additional risks and uncertainties, including the increased possibility of litigation and regulatory inquiries. Any of the foregoing may affect investor confidence in the accuracy of our financial disclosures and may raise reputational risks for our business, both of which could harm our business and financial results.
We have never declared or paid dividends on our common stock and we do not anticipate paying dividends in the foreseeable future.
We have never declared or paid cash dividends on our common stock. For the foreseeable future, we currently intend to retain all available funds and any future earnings to support our operations and to finance the growth and development of our business. Any future determination to declare cash dividends will be made at the discretion of our Board, subject to compliance with applicable laws and covenants under current or future credit facilities, which may restrict or limit our ability to pay dividends, and will depend on our financial condition,
68
Table of Contents
operating results, capital requirements, general business conditions and other factors that our Board may deem relevant. We do not anticipate paying any cash dividends on our common stock in the foreseeable future. As a result, a return on your investment in our securities will only occur if the price of our common stock appreciates.
Future sales and issuances of our common stock or rights to purchase common stock could result in substantial dilution to the percentage ownership of our stockholders.
We expect that significant additional capital will be needed in the future to continue our planned operations. To raise capital, we may sell common stock or other securities convertible into or exchanged for our common stock in one or more transactions, and in a manner we determine from time to time and at prices that may not be the same as the price per share paid by other investors, and dilution to our stockholders could result. The price per share at which we sell additional shares of our common stock, or securities convertible or exchangeable into common stock, in future transactions may be higher or lower than the price per share paid by other investors. New investors could also receive rights, preferences and privileges senior to those of existing holders of our common stock. In addition, in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock, we may be required to proportionally adjust the conversion price, exercise price or number of shares issuable upon exercise of any outstanding warrants.
Our corporate governance structure may prevent our acquisition by another company at a premium over the public trading price of our shares.
It is possible that the acquisition of a majority of our outstanding voting stock by another company could result in our stockholders receiving a premium over the public trading price for our shares. Provisions of our restated certificate of incorporation and our amended and restated bylaws, each as amended, and of Delaware corporate law could delay or make more difficult an acquisition of our company by merger, tender offer or proxy contest, even if it would create an immediate benefit to our stockholders. Furthermore, our certificate of incorporation also provides for a classified board of directors with directors divided into three classes serving staggered terms. These provisions may have the effect of delaying or preventing a change in control of us without action by our stockholders and, therefore, could adversely affect the price of our stock or the possibility of sale of shares to an acquiring person.
We have a limited number of unreserved, authorized shares.
If we seek equity financing we may need to use a significant percentage of our unreserved authorized shares of common stock in such an offering, and would therefore need stockholder approval to implement an increase in our authorized shares of common stock or a reverse stock split in order to issue additional shares of common stock in the future. Our certificate of incorporation and the Delaware General Corporation Law, or the DGCL, currently require the approval of stockholders holding not less than a majority of all outstanding shares of capital stock entitled to vote in order to approve an increase in our authorized shares of common stock or a reverse stock split. There are no assurances that stockholder approval will be obtained, in which event we will be unable to raise additional capital through the issuance of shares of common stock to fund our future operations.
Although we are required to use our reasonable best efforts to have an effective registration statement covering the issuance of shares of common stock underlying certain of our outstanding warrants at the time that holders of our warrants exercise their warrants, we cannot guarantee that a registration statement will be effective, in which case holders of our warrants are entitled to a cashless exercise of their warrants.
Pursuant to the terms of certain of our warrants, we are obligated to have an effective registration statement covering the resale of the shares of common stock underlying such warrants. If no registration is effective at the time a warrant holder seeks to exercise their warrants, we would be obligated to issue shares to such warrant holder in a “cashless exercise” in exchange for such holder’s warrants, in which case we would not receive the cash that we would otherwise receive in an exercise of warrants for cash.
69
Table of Contents
| • | Mechanisms, controls, and technologies designed to prevent or mitigate system intrusion or data loss, theft, misuse, or other security incidents or vulnerabilities and maintain a stable and secure information technology environment. |
| • | Information security policies, network and device security, encryption standards, risk management, as well as security tools such as malware protection and secure authentication tools. |
| • | We conduct ongoing monitoring of critical systems for any compromised or potentially compromised accounts, and conduct regular trainings for our employees and senior management on cyber and information security. |
Table of Contents
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Our common stock trades on Nasdaq under the symbol “BNTC.” On September 18, 2025, the closing sale price of our common stock as reported on Nasdaq was $13.99 per share.
Holders
As of September 15, 2025, we had approximately 1,128 record holders of our common stock. The number of record holders is based on the actual number of holders registered on the books of our transfer agent and does not reflect holders of shares in “street name” or persons, partnerships, associations, corporations or other entities identified in security position listings maintained by depository trust companies.
Dividends
We never have declared or paid any cash dividends on our capital stock. Currently, we anticipate that we will retain all available funds for use in the operation and expansion of our business and do not anticipate paying any cash dividends for the foreseeable future. Any future determination relating to dividend policy will be made at the discretion of our Board and will depend on our future earnings, capital requirements, financial condition, prospects, applicable Delaware law, which provides that dividends are only payable out of surplus or current net profits, and other factors that our Board deems relevant.
Recent Sales of Unregistered Securities
None.
Use of Proceeds
Not applicable.
Purchases of Equity Securities
Not applicable.
Item 6. Reserved.
71
Table of Contents
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
You should read the following discussion and analysis of financial condition and operating results together with our consolidated financial statements and the related notes and other financial information included in Item 8 in this Annual Report. This discussion contains forward-looking statements that involve risks and uncertainties. As a result of many factors, such as those set forth in the section of the Annual Report captioned “Risk Factors” and elsewhere in this Annual Report, our actual results may differ materially from those anticipated in these forward- looking statements.
Restatement of Prior Period Financial Statements
We have restated our previously issued unaudited condensed consolidated financial statements for the quarterly periods and year-to-date periods ended March 31, 2025 and December 31, 2024, as contained in this Annual Report on Form 10-K. Refer to the “Explanatory Note” Preceding Item 1, Business, for background on the restatement, the periods impacted, control considerations, and other information. In addition, we have restated certain previously reported financial information for the quarterly periods and year-to-date periods ended March 31, 2025 and December 31, 2024 in this Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations, including but not limited to information within the Results of Operations section. See Note 3, Restatement of Prior Period Financial Statements, in the notes to the consolidated financial statements in this Annual Report on Form 10-K, for additional information related to the restatement, including descriptions of the misstatements and the impacts on our consolidated financial statements.
Overview
We endeavor to become the leader in discovery, development, and commercialization of therapeutic agents capable of addressing significant unmet medical need via the application of the silence and replace approach to the treatment of genetic disorders.
Benitec Biopharma Inc. (“Benitec” or the “Company” or in the third person, “we” or “our”) is a clinical-stage biotechnology company focused on the advancement of novel genetic medicines with headquarters in Hayward, California. The proprietary platform, called DNA-directed RNA interference, or ddRNAi, combines RNA interference, or RNAi, with gene therapy to create medicines that facilitate sustained silencing of disease-causing genes following a single administration. The unique therapeutic constructs also enable the simultaneous delivery of wildtype replacement genes, facilitating the proprietary “silence and replace” approach to the treatment of genetically defined diseases. We are developing a silence and replace-based therapeutic (BB-301) for the treatment of Oculopharyngeal Muscular Dystrophy (OPMD), a chronic, life-threatening genetic disorder.
BB-301 is a silence and replace-based genetic medicine currently under development by Benitec. BB-301 is an AAV-based gene therapy designed to permanently silence the expression of the disease-causing gene (to slow, or halt, the biological mechanisms underlying disease progression in OPMD) and to simultaneously replace the mutant gene with a wildtype gene (to drive restoration of function in diseased cells). This fundamental therapeutic approach to disease management is called “silence and replace.” The silence and replace mechanism offers the potential to restore the normative physiology of diseased cells and tissues and to improve treatment outcomes for patients suffering from the chronic, and potentially fatal, effects of OPMD. BB-301 has been granted Orphan Drug Designation in the United States and the European Union.
The targeted gene silencing effects of RNAi, in conjunction with the durable transgene expression achievable via the use of modified viral vectors, imbues the silence and replace approach with the potential to produce permanent silencing of disease-causing genes along with simultaneous replacement of the wild type gene function following a single administration of the proprietary genetic medicine. We believe that this novel mechanistic profile of the current and future investigational agents developed by Benitec could facilitate the achievement of robust and durable clinical activity while greatly reducing the frequency of drug administration traditionally expected for medicines employed for the management of chronic diseases. Additionally, the
72
Table of Contents
achievement of permanent gene silencing and gene replacement may significantly reduce the risk of patient non-compliance during the course of medical management of potentially fatal clinical disorders.
Royalties, milestone payments and other license fees
We have been and in the future may be required to pay royalties, milestone payments and other license fees in connection with our licensing of intellectual property from third parties, including as discussed below.
Foreign Currency Translation and Other Comprehensive Income (Loss)
The Company’s functional currency and reporting currency is the United States dollar. BBL’s functional currency is the Australian dollar (AUD). Assets and liabilities are translated at the exchange rate in effect at the balance sheet date. Revenues and expenses are translated at the average rate of exchange prevailing during the reporting period. Equity transactions are translated at each historical transaction date spot rate. Translation adjustments arising from the use of different exchange rates from period to period are included as a component of stockholders’ equity as “Accumulated other comprehensive income (loss).” Gains and losses resulting from foreign currency translation are included in the consolidated statements of operations and comprehensive income (loss) as other comprehensive income (loss).
Financing and Financing-Related Transactions During the Year Ended June 30, 2025
ATM Agreement
On October 11, 2024, we entered into a Sales Agreement (the “Sales Agreement”) with Leerink Partners LLC (the “Agent”). Pursuant to the terms of the Sales Agreement, we may offer and sell shares of our common stock having an aggregate offering amount of up to $75 million from time to time through the Agent. The Agent will use its commercially reasonable efforts, as the agent and subject to the terms of the Sales Agreement, to sell the shares offered. Sales of the shares, if any, may be made in sales deemed to be an “at-the-market offering” as defined in Rule 415 under the Securities Act of 1933, as amended. We may also agree to sell shares to the Agent as principal for its own account on terms agreed to by us and the Agent. The Agent will be entitled to a commission from us of 3.0% of the gross proceeds from the sale of shares sold under the Sales Agreement. In addition, we have agreed to reimburse certain expenses incurred by the Agent in connection with the offering. Shares sold pursuant to the Sales Agreement, if any, will be sold pursuant to our shelf registration statement on Form S-3 (File No. 333-277310), that was filed with the Securities and Exchange Commission, including the related prospectus, dated March 5, 2024, as supplemented by a prospectus supplement. As of June 30, 2025, we have not sold any shares of common stock pursuant to the Sales Agreement.
March 2025 Capital Raise
On March 25, 2025, we entered into an Underwriting Agreement with Leerink Partners LLC and TD Securities (USA) LLC, as representatives of the several underwriters named therein, pursuant to which we agreed to issue and sell, in an underwritten offering by us (the “Underwritten Offering”), (i) 1,143,000 shares of our common stock, par value $0.0001 per share (the “Common Stock”) at a purchase price to investors of $13.00 per share, and (ii) pre-funded warrants to purchase 300,000 shares of Common Stock at an exercise price of $0.0001 per share at a purchase price to investors of $12.999 per warrant. In connection with the Underwritten Offering, we entered into a Securities Purchase Agreement with entities affiliated with each of Suvretta Capital, a greater than 5% beneficial owner prior to the offering (together, the “Purchasers”), pursuant to which we agreed to issue and sell to the Purchasers an aggregate of 900,000 shares of Common Stock at a purchase price of $13.00 per share in a registered direct offering (the “Direct Offering,” and together with the Underwritten Offering, the “Offerings”), the same price per share as the offering price in the Underwritten Offering. We received gross proceeds of approximately $30.5 million and net proceeds of approximately $28.2 million from the Offerings.
73
Table of Contents
Results of Operations
Quarterly Financial Information (Unaudited)
As a result of the restatement described above, we are restating our comparisons of expenses for the impacted periods as follows:
Operating Expenses
The following table sets forth a summary of our expenses for each of the periods:
| Three Months Ended March 31, |
Nine Months Ended March 31, |
|||||||||||||||
| 2025 | 2024 | 2025 | 2024 | |||||||||||||
| (US$’000) | ||||||||||||||||
| Operating Expenses: |
||||||||||||||||
| Royalties and License Fees |
$ | — | $ | (3 | ) | $ | — | $ | (108 | ) | ||||||
| Research and development (as restated) |
6,495 | 2,566 | 15,465 | 12,097 | ||||||||||||
| General and administrative (as restated) |
8,840 | 1,578 | 16,466 | 4,953 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses (as restated) |
$ | 15,335 | $ | 4,141 | $ | 31,931 | $ | 16,942 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
During the three and nine months ended March 31, 2025, respectively, we incurred $6.5 million and $15.5 million in research and development expenses, respectively, as compared to $2.6 million and $12.1 million for the comparable periods ended March 31, 2024. Research and development expenses relate primarily to ongoing clinical development of BB-301 for the treatment of OPMD. The year-over-year increase for the three and nine months ended March 31, 2025, reflects the timing of contract manufacturing activity and the timing of payments for the OPMD Natural History and Dosing study.
General and administrative expense totaled $8.8 million and $16.5 million for the three and nine months ended March 31, 2025, compared to $1.6 million and $5.0 million for the comparable periods ended March 31, 2024. The increase for the three month period, 2025 relates primarily to an increase in share-based compensation of $7.4 million, travel expenses of $176 thousand and salaries and wages of $254 thousand. The increase in the nine month period relates to an increase in share-based compensation of $10.9 million, and higher corporate costs related to an increase in legal fees, and higher travel expenses as well as an increase in salaries and wages.
The following table sets forth a summary of our expenses for each of the periods:
| Three Months Ended December 31, |
Six Months Ended December 31, |
|||||||||||||||
| 2024 | 2023 | 2024 | 2023 | |||||||||||||
| (US$’000) | ||||||||||||||||
| Operating Expenses: |
||||||||||||||||
| Royalties and License Fees |
— | $ | 1 | — | $ | (105 | ) | |||||||||
| Research and development (as restated) |
5,385 | 5,102 | 8,970 | 9,531 | ||||||||||||
| General and administrative (as restated) |
5,420 | 1,824 | 7,626 | 3,375 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses (as restated) |
$ | 10,805 | $ | 6,927 | $ | 16,596 | $ | 12,801 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
During the three and six months ended December 31, 2024, respectively, we incurred $5.4 million and $9.0 million in research and development expenses, respectively, as compared to $5.1 million and $9.5 million for the comparable periods ended December 31, 2023. Research and development expenses relate primarily to ongoing clinical development of BB-301 for the treatment of OPMD. The year-over-year decrease for the three and
74
Table of Contents
six-month periods ended December 31, 2024 reflects the timing of contract manufacturing activity and the timing of payments for the OPMD Natural History and Dosing study.
General and administrative expense totaled $5.4 million and $7.6 million for the three and six months ended December 31, 2024, compared to $1.8 million and $3.4 million for the comparable periods ended December 31, 2023. The increase for the three and six-month period ended December 31, 2024 relates primarily to an increase in share-based compensation of $2.7 million and $3.4 million and corporate costs related to the filing of an At-the-Market offering and related legal fees of $643 thousand and $945 thousand, respectively.
Years Ended June 30, 2025 and 2024
Revenues
We did not generate or recognize any revenue during the years ended June 30, 2025 and 2024.
Royalties and License Fees
Royalties and license fees consist primarily of payments we are required to remit for royalties and other payments related to in-licensed intellectual property. We cannot precisely predict the amount, if any, of royalties we will owe in the future, and if our calculations of royalty payments are incorrect, we may owe additional royalties, which could negatively affect our results of operations. Furthermore, we may enter into additional license agreements in the future, which may also include royalty, milestone, and other payments.
Research and Development Expenses
Research and development expenses relate primarily to the cost of conducting clinical and preclinical trials. Preclinical and clinical development costs are a significant component of research and development expenses. We record accrued liabilities for estimated costs of research and development activities conducted by third-party service providers, which include the conduct of preclinical studies and clinical trials, and contract manufacturing activities. We record the estimated costs of research and development activities based upon the estimated amount of services provided but not yet invoiced and includes these costs in trade and other payables on the consolidated balance sheets and within research and development expenses on the consolidated statements of operations and comprehensive loss.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries, related benefits, travel, and equity-based compensation expense. General and administrative expenses also include facility expenses, professional fees for legal, consulting, accounting and audit services and other related costs.
We anticipate that our general and administrative expenses may increase as we focus on the continued development of the clinical OPMD program. We also anticipate an increase in expenses relating to accounting, legal and regulatory-related services associated with maintaining compliance with exchange listing and SEC requirements, director and officer insurance premiums and other similar costs.
Ms. Boston was appointed as our Chief Financial Officer effective January 1, 2025. On December 9, 2024, the Compensation Committee approved increases of the base salaries of Dr. Jerel Banks and Megan Boston to $667,000 and $415,000, respectively, each adjustment being effective as of January 1, 2025. The Compensation Committee further determined that the target annual discretionary bonus with respect to our 2025 fiscal year for Dr. Jerel Banks and Megan Boston will be 55% and 40% of their base salary, respectively. On December 9, 2024, the Board of Directors appointed Sophie Mukadam as Chief Operating Officer, effective as of January 1, 2025. Sophie Mukadam will receive a base salary of $500,000 and a target annual bonus of 40% of base salary. On February 13, 2025, the Compensation Committee of our board of directors approved a change to the base salary of Megan Boston, CFO, from USD$415,000 to USD$531,900 to be effective March 1, 2025. The base salary change was approved in anticipation of Ms. Boston relocating from Australia to Los Angeles, California.
75
Table of Contents
In connection with such relocation, the Compensation Committee also approved moving and transitional housing allowances in aggregate amounts of approximately $5,000 and $18,000, respectively.
Operating Expenses
The following table sets forth a summary of our expenses for each of the periods:
| Year Ended June 30, | ||||||||
| 2025 | 2024 | |||||||
| (US$’000) | ||||||||
| Expenses: |
||||||||
| Royalties and license fees |
$ | — | $ | (108 | ) | |||
| Research and development |
18,332 | 15,609 | ||||||
| General and administrative |
23,433 | 6,989 | ||||||
|
|
|
|
|
|||||
| Total expenses |
$ | 41,765 | $ | 22,490 | ||||
|
|
|
|
|
|||||
During the years ended June 30, 2025 and 2024, we incurred royalties and license fees expenses of zero and $(108) thousand, respectively. The credits to expense during the year ended June 30, 2024, relates to the reversal of accruals for license fees no longer due.
During the year ended June 30, 2025, we incurred $18.3 million in research and development expenses, as compared to $15.6 million for the comparable year ended June 30, 2024. Research and development expenses relate primarily to ongoing clinical development of BB-301 for the treatment of OPMD. The year-over-year increase for the year ended June 30, 2025, reflects the timing of contract manufacturing activity and the timing of payments for the OPMD Natural History and Dosing study.
General and administrative expenses totaled $23.4 million for the year ended June 30, 2025, compared to $7.0 million for the comparable year ended June 30, 2024. The increase for the year ended June 30, 2025, relates primarily to increases in share-based compensation of $14.5 million, legal fees of $492 thousand, consulting fees of $605 thousand, travel expenses of $219 thousand, and salaries and wages of $685 thousand.
Other Income (Expense)
The following table sets forth a summary of our other income (loss) for each of the periods:
| Year Ended June 30, | ||||||||
| 2025 | 2024 | |||||||
| (US$’000) | ||||||||
| Other Loss: |
||||||||
| Foreign currency transaction gain (loss) |
$ | (71 | ) | $ | 40 | |||
| Interest income, net |
3,286 | 904 | ||||||
| Other expense, net |
(131 | ) | (204 | ) | ||||
| Gain on extinguishment of liabilities |
764 | — | ||||||
| Unrealized loss on investment |
— | (1 | ) | |||||
|
|
|
|
|
|||||
| Total other income (loss), net |
$ | 3,848 | $ | 739 | ||||
|
|
|
|
|
|||||
Other income (loss), net during the year ended June 30, 2025, which consists of foreign currency transaction gain (loss), interest income other expense, net, gain on extinguishment of liabilities, and unrealized loss on investment, totaled $3,848 thousand. Other income (loss), net during the year ended June 30, 2024, which consists of foreign currency transaction gain (loss), interest expense, other income (expense), and unrealized loss on investment, totaled $739. Foreign currency transaction gains and losses reflect changes in foreign exchange
76
Table of Contents
rates. Net interest income increased for year ended June 30, 2025, in comparison the year ended June 30, 2024, reflects the increase in our cash and cash equivalent balances. Other expense, net recognized during the years ended June 30, 2025 and 2024 relate to recognition of a franchise tax expenses. Gain on extinguishment of liabilities is due to us settling outstanding trade payables and accrued clinical development project costs of $1.2 million with a vendor for $495 thousand due to a contractual dispute regarding contract performance and deliverables. This settlement resulted in a gain of $764 thousand in the current year.
Liquidity and Capital Resources
We have incurred cumulative losses and negative cash flows from operations since our predecessor’s inception in 1995. We had accumulated losses of $218 million as of June 30, 2025. We expect that our research and development expenses will increase due to the continued development of the OPMD program. It is also likely that there will be an increase in the general and administrative expenses due to the obligations of being a domestic public company in the United States.
We had no borrowings as of June 30, 2025 and do not currently have a credit facility. As of June 30, 2025, we had outstanding warrants to purchase 20,443,496 shares of Common Stock consisting of the following:
| June 30, 2025 |
June 30, 2024 |
|||||||
| Purchase Warrants to purchase Common Stock |
— | 6,300 | ||||||
| September 2022 Pre-Funded Warrants to purchase Common Stock |
588,236 | 588,236 | ||||||
| Series 2 Warrants to purchase Common Stock |
101,537 | 1,655,464 | ||||||
| August 2023 Pre-Funded Warrants to purchase Common Stock |
12,179,739 | 14,172,919 | ||||||
| Common Warrants to purchase Common Stock |
5,071,148 | 15,263,988 | ||||||
| April 2024 Pre-Funded Warrants to purchase Common Stock |
2,202,836 | 2,584,239 | ||||||
| March 2025 Pre-Funded Warrants to purchase Common Stock |
300,000 | — | ||||||
|
|
|
|
|
|||||
| Total |
20,443,496 | 34,271,146 | ||||||
|
|
|
|
|
|||||
As of June 30, 2025, we had cash and cash equivalents of approximately $97.7 million. Cash in excess of immediate requirements is invested in accordance with our investment policy, primarily with a view to liquidity and capital preservation. Currently, our cash and cash equivalents are held in bank accounts. On October 11, 2024, we entered into the Sales Agreement as discussed above, which provides for the sale of up to $75 million of our common stock from time-to-time in “at-the-market offerings”. On March 25, 2025, we completed a financing which raised $30.5 million.
The following table sets forth a summary of the net cash flow activity for each of the periods set forth below:
| Year Ended June 30, |
||||||||
| 2025 | 2024 | |||||||
| (US$’000) | ||||||||
| Net cash provided by (used in): |
||||||||
| Operating activities |
$ | (23,588 | ) | $ | (19,403 | ) | ||
| Investing activities |
(18 | ) | (179 | ) | ||||
| Financing activities |
70,485 | 68,029 | ||||||
| Effects of exchange rate changes on cash and cash equivalents |
49 | (8 | ) | |||||
|
|
|
|
|
|||||
| Net increase in cash, cash equivalents, and restricted cash |
$ | 46,928 | $ | 48,439 | ||||
|
|
|
|
|
|||||
77
Table of Contents
Operating activities
Net cash used in operating activities for the years ended June 30, 2025 and 2024 was $23.6 million and $19.4 million, respectively. Net cash used in operating activities was primarily the result of our net loss, partially offset by non-cash expenses, and changes in working capital, including a decrease in payables and trade and other receivables and increases in prepaids.
Investing activities
Net cash used in investing activities for the years ended June 30, 2025 and 2024 was $18 thousand and $179 thousand, respectively. Cash used in investing activities in the years ended June 30, 2025 and 2024 was related to the purchase of furniture and fixtures and lab equipment, respectively.
Financing activities
Net cash provided by financing activities was $70.5 million and $68.0 million for the years ended June 30, 2025 and 2024, respectively. Cash from financing activities in the year ended June 30, 2025 was related to the issuance of common stock from the exercise of pre-funded warrants, Series 2 warrants, and common warrants, and an underwritten and direct offering with net proceeds of $72.8 million, partially offset by $2.3 million in share issuance costs. Cash from financing activities in the year ended June 30, 2024 was related to the issuance of common stock, pre-funded warrants, and common warrants, with gross proceeds of $73.9 million, partially offset by $5.9 million in share issuance costs.
Funding Requirements
The future of us as an operating business will depend on its ability to manage operating costs and budgeted amounts and obtain adequate financing.
We do not have any products approved for sale and have not generated any revenue from product sales. We do not know when, or if, we will generate any revenue from product sales. We do not expect to generate significant revenue from product sales unless and until we obtain regulatory approval of and commercialize one of our current or future product candidates.
Unless and until we establish significant revenues from licensing programs, strategic alliances or collaboration arrangements with pharmaceutical companies, or from product sales, we anticipate that we will continue to generate losses for the foreseeable future, and we expect the losses to increase as we continue the development of product candidates and begin to prepare to commercialize any product that receives regulatory approval. We are subject to the risks inherent in the development of new gene therapy products, and we may encounter unforeseen expenses, difficulties, complications, delays, and other unknown factors that may adversely affect our business. We estimate that our cash and cash equivalents will be sufficient to fund our operations for at least the next twelve months from the date of this report.
We have based our projections of operating capital requirements on assumptions that may prove to be incorrect and we may use all of our available capital resources sooner than we expect. Because of the numerous risks and uncertainties associated with research, development, and commercialization of pharmaceutical products, we are unable to estimate the exact amount of our operating capital requirements. Our future funding requirements will depend on many factors, including, but not limited to:
| • | the timing and costs of our clinical trials for our ddRNAi and silence and replace product candidates; |
| • | the timing and costs of our preclinical studies for our ddRNAi and silence and replace product candidates; |
| • | the number and characteristics of product candidates that we pursue; |
78
Table of Contents
| • | the outcome, timing, and costs of seeking regulatory approvals; |
| • | revenue received from commercial sales of any of our product candidates that may receive regulatory approval; |
| • | the terms and timing of any future collaborations, licensing, consulting, or other arrangements that we may establish; |
| • | the amount and timing of any payments we may be required to make, or that we may receive, in connection with the licensing, filing, prosecution, defense and enforcement of any patents or other intellectual property rights; |
| • | the costs of preparing, filing and prosecuting patent applications, maintaining and protecting our intellectual property rights and defending against intellectual property related claims; and |
| • | the extent to which we need to in-license or acquire other products and technologies. |
Contractual Obligations and Commercial Commitments
On October 1, 2016, we entered into an operating lease for office space in Hayward, California that originally expired in April 2018. We have entered into lease amendments that extended the lease through December 2027. We also entered into a new lease in Los Angeles, California, which has an initial expiration date in July 2026. See Note 9 of the Notes to Consolidated Financial Statements included in this Annual Report on Form 10-K.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined under SEC rules.
We enter into contracts in the normal course of business with third-party contract research organizations, contract development and manufacturing organizations and other service providers and vendors. These contracts generally provide for termination on notice and, therefore, are cancellable contracts and not considered contractual obligations and commitments.
Critical Accounting Policies and Significant Accounting Estimates
The preparation of consolidated financial statements and related disclosures in conformity with accounting principles generally accepted in the United States of America requires us to make judgments, assumptions and estimates that affect the amounts reported. Note 2 of the Notes to Consolidated Financial Statements included in this Annual Report on Form 10-K describes the significant accounting policies used in the preparation of the consolidated financial statements. Certain of these significant accounting policies are considered to be critical accounting policies.
A critical accounting policy is defined as one that is both material to the presentation of our consolidated financial statements and requires us to make difficult, subjective, or complex judgments that could have a material effect on our financial condition or results of operations. Specifically, these policies have the following attributes: (1) we are required to make assumptions about matters that are highly uncertain at the time of the estimate; and (2) different estimates we could reasonably have used, or changes in the estimate that are reasonably likely to occur, would have a material effect on our financial condition or results of operations.
Estimates and assumptions about future events and their effects cannot be determined with certainty. We base our estimates on historical experience and on various other assumptions believed to be applicable and reasonable under the circumstances. These estimates may change as new events occur, as additional information is obtained and as our operating environment changes. These changes have historically been minor and have been included
79
Table of Contents
in the consolidated financial statements as soon as they became known. In addition, we are periodically faced with uncertainties, the outcomes of which are not within its control and will not be known for prolonged periods of time. These uncertainties are discussed in the section above entitled “Risk Factors.” Based on a critical assessment of its accounting policies and the underlying judgments and uncertainties affecting the application of those policies, we believe that our consolidated financial statements are fairly stated in accordance with accounting principles generally accepted in the United States of America and provide a meaningful presentation of our financial condition and results of operations.
We believe that the following are critical accounting policies:
Research and Development Expense
Preclinical and clinical trial costs are a significant component of our research and development expenses. We accrue for preclinical and clinical development costs based on factors such as estimates of the work completed and in accordance with agreements established with our third-party service providers. We make significant judgments and estimates in determining the accrued liabilities balance at the end of each reporting period. As actual costs become known, we adjust our accrued liabilities accordingly on a prospective basis and will do so in the period in which the facts that give rise to the revision become reasonably certain.
Share-based Compensation Expense
We record share-based compensation in accordance with ASC 718, Stock Compensation. ASC 718 requires the fair value of all share-based employee compensation awarded to employees and non-employees to be recorded as an expense over the shorter of the service period or the vesting period. We determine employee and non-employee share-based compensation based on grant-date fair value using the Black-Scholes Option Pricing Model and allocate the resulting compensation expense over the corresponding requisite service period using the graded vesting attribution method. We account for forfeitures of share-based awards as they occur.
Recent Accounting Pronouncements
For a discussion of recent accounting pronouncements that we have adopted and have not yet adopted, see Note 2 to our consolidated financial statements.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk.
We are a smaller reporting company and not required to provide this information.
80
Table of Contents
F-2 |
||||
F- 3 |
||||
F- 4 |
||||
F- 5 |
||||
F- 6 |
||||
F- 7 |
June 30, 2025 |
June 30, 2024 |
|||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | $ | ||||||
| Restricted cash |
||||||||
| Trade and other receivables |
||||||||
| Prepaid and other assets |
||||||||
| |
|
|
|
|||||
| Total current assets |
||||||||
| Property and equipment, net |
||||||||
| Deposits |
||||||||
| Prepaid and other assets |
||||||||
| Right-of-use |
||||||||
| |
|
|
|
|||||
| Total assets |
$ | $ | ||||||
| |
|
|
|
|||||
| Liabilities and stockholders’ equity |
||||||||
| Current liabilities: |
||||||||
| Trade and other payables |
$ | $ | ||||||
| Accrued employee benefits |
||||||||
| Lease liabilities, current portion |
||||||||
| |
|
|
|
|||||
| Total current liabilities |
||||||||
| |
|
|
|
|||||
| Non-current accrued employee benefits |
||||||||
| Lease liabilities, less current portion |
||||||||
| |
|
|
|
|||||
| Total liabilities |
||||||||
| |
|
|
|
|||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $ |
||||||||
| Common stock, $ |
||||||||
| Additional paid-in capital |
||||||||
| Accumulated deficit |
( |
) | ( |
) | ||||
| Accumulated other comprehensive loss |
( |
) | ( |
) | ||||
| |
|
|
|
|||||
| Total stockholders’ equity |
||||||||
| |
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | $ | ||||||
| |
|
|
|
|
|
|
|
|
Year Ended |
||||||||
June 30, |
||||||||
2025 |
2024 |
|||||||
| Operating expenses |
||||||||
| Royalties and license fees |
$ | $ | ( |
) | ||||
| Research and development |
||||||||
| General and administrative |
||||||||
| |
|
|
|
|||||
| Total operating expenses |
||||||||
| |
|
|
|
|||||
| Loss from operations |
( |
) | ( |
) | ||||
| Other income (loss): |
||||||||
| Foreign currency transaction gain (loss) |
( |
) | ||||||
| Interest income (expense), net |
||||||||
| Other expense, net |
( |
) | ( |
) | ||||
| Gain on extinguishment of liabilities |
||||||||
| Unrealized loss on investment |
( |
) | ||||||
| |
|
|
|
|||||
| Total other income (loss), net |
||||||||
| |
|
|
|
|||||
| Net loss |
$ | ( |
) | $ | ( |
) | ||
| |
|
|
|
|||||
| Other comprehensive income: |
||||||||
| Unrealized foreign currency translation gain (loss) |
( |
) | ||||||
| |
|
|
|
|||||
| Total other comprehensive income (loss) |
( |
) | ||||||
| |
|
|
|
|||||
| Total comprehensive loss |
$ | ( |
) | $ | ( |
) | ||
| |
|
|
|
|||||
| Net loss |
$ | ( |
) | $ | ( |
) | ||
| |
|
|
|
|||||
| Deemed dividends |
( |
) | ||||||
| |
|
|
|
|||||
| Net loss attributable to common shareholders |
$ | ( |
) | $ | ( |
) | ||
| |
|
|
|
|||||
| Net loss per share: |
||||||||
| Basic and diluted |
$ | ( |
) | $ | ( |
) | ||
| |
|
|
|
|||||
| Weighted average number of shares outstanding: basic and diluted |
||||||||
Common Stock |
Additional Paid-in Capital |
Accumulated Deficit |
Accumulated Other Comprehensive Loss |
Total Stockholders’ Equity |
||||||||||||||||||||
Shares |
Amount |
|||||||||||||||||||||||
Balance at June 30, 2023 |
$ | $ | $ | ( |
) | $ | ( |
) | $ | |||||||||||||||
Issuance of common stock, pre-funded warrants, andcommon warrants sold for cash, net of offering costs of $ |
— | — | ||||||||||||||||||||||
Issuance of common stock and pre-funded warrants sold for cash, net of offering costs of $ |
— | — | ||||||||||||||||||||||
Exercise of pre-funded warrants |
— | — | — | — | — | |||||||||||||||||||
Exercise of Series 2 warrants |
— | — | — | |||||||||||||||||||||
Exercise of common warrants |
— | — | — | |||||||||||||||||||||
Anti-dilution adjustment to warrants |
— | — | ( |
) | — | — | ||||||||||||||||||
Share-based compensation |
— | — | — | — | ||||||||||||||||||||
Foreign currency translation loss |
— | — | — | — | ( |
) | ( |
) | ||||||||||||||||
Net loss |
— | — | — | ( |
) | — | ( |
) | ||||||||||||||||
Balance at June 30, 2024 |
$ | $ | $ | ( |
) | $ | ( |
) | $ | |||||||||||||||
Issuance of common stock and pre-funded warrants sold for cash, net of offering costs of $ |
— | — | ||||||||||||||||||||||
Exercise of pre-funded warrants |
— | — | — | — | — | |||||||||||||||||||
Exercise of Series 2 warrants |
— | — | — | |||||||||||||||||||||
Exercise of common warrants, net of offering cost of $ |
— | — | — | |||||||||||||||||||||
Share-based compensation |
— | — | — | — | ||||||||||||||||||||
Foreign currency translation gain |
— | — | — | — | ||||||||||||||||||||
Net loss |
— | — | — | ( |
) | — | ( |
) | ||||||||||||||||
Balance at June 30, 2025 |
$ | $ | $ | ( |
) | $ | ( |
) | $ | |||||||||||||||
| Year Ended June 30, |
||||||||
2025 |
2024 |
|||||||
Cash flows from operating activities: |
||||||||
Net loss |
$ | ( |
) | $ | ( |
) | ||
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
Depreciation and amortization |
||||||||
Amortization of right-of-use |
||||||||
Unrealized loss on investment |
— | |||||||
Gain on extinguishment of liabilities |
( |
) | — | |||||
Share-based compensation expense |
||||||||
Changes in operating assets and liabilities: |
||||||||
Trade and other receivables |
( |
) | ||||||
Prepaid and other assets |
( |
) | ||||||
Trade and other payables |
( |
) | ||||||
Accrued employee benefits |
( |
) | ||||||
Lease liabilities |
( |
) | ( |
) | ||||
Net cash used in operating activities |
( |
) | ( |
) | ||||
Cash flows from investing activities: |
||||||||
Purchase of property and equipment |
( |
) | ( |
) | ||||
Net cash used in investing activities |
( |
) | ( |
) | ||||
Cash flows from financing activities: |
||||||||
Proceeds from issuance of common stock, pre-funded warrants, and common warrants |
||||||||
Proceeds from exercise of pre-funded warrants, Series 2 warrants common warrants |
||||||||
Share and pre-funded warrants issuance transaction costs |
( |
) | ( |
) | ||||
Net cash provided by financing activities |
||||||||
Effects of exchange rate changes on cash, cash equivalents, and restricted cash |
( |
) | ||||||
Net increase in cash, cash equivalents, and restricted cash |
||||||||
Cash, cash equivalents, and restricted cash, beginning of period |
||||||||
Cash, cash equivalents, and restricted cash, end of period |
$ | $ | ||||||
Supplemental disclosure of cash flow information |
||||||||
Initial measurement of operating lease right-of-use assets |
$ | $ | ||||||
| |
|
|
|
|||||
Re-measurement of operating lease right-of-use assets |
$ | $ | ||||||
| |
|
|
|
|||||
Deemed dividend |
$ | $ | ||||||
| |
|
|
|
|||||
| Principal place of business/country of incorporation | ||
Benitec Biopharma Proprietary Limited (“BBL”) |
||
Benitec Australia Proprietary Limited |
||
Benitec Limited |
||
Benitec, Inc. |
||
Benitec LLC |
||
RNAi Therapeutics, Inc. |
||
Tacere Therapeutics, Inc. |
||
Benitec IP Holdings, Inc. |
June 30, 2025 |
June 30, 2024 |
|||||||
Exchange rate on balance sheet dates |
||||||||
USD: AUD Exchange Rate |
||||||||
Average exchange rate for the period |
||||||||
USD: AUD Exchange Rate |
||||||||
Level 1: |
Observable inputs such as quoted prices (unadjusted) in active markets for identical assets or liabilities. | |
Level 2: |
Inputs, other than quoted prices that are observable, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active. | |
Level 3: |
Unobservable inputs in which little or no market data exists, therefore developed using estimates and assumptions developed by us, which reflect those that a market participant would use. | |
| Software | ||
| Lab equipment | ||
| Computer hardware | ||
| Leasehold improvements |
As of March 31, 2025 |
||||||||||||
As Reported |
Adjustment |
As Restated |
||||||||||
Assets |
||||||||||||
Current assets: |
||||||||||||
Cash and cash equivalents |
$ | $ | $ | |||||||||
Restricted cash |
||||||||||||
Trade and other receivables |
||||||||||||
Prepaid and other assets |
||||||||||||
Total current assets |
||||||||||||
Property and equipment, net |
||||||||||||
Deposits |
||||||||||||
Prepaid and other assets |
||||||||||||
Right-of-use assets |
||||||||||||
Total assets |
$ | $ | $ | |||||||||
Liabilities and stockholders’ equity |
||||||||||||
Current liabilities: |
||||||||||||
Trade and other payables |
$ | $ | $ | |||||||||
Accrued employee benefits |
||||||||||||
Lease liabilities, current portion |
||||||||||||
Total current liabilities |
||||||||||||
Non-current accrued employee benefits |
||||||||||||
Lease liabilities, less current portion |
||||||||||||
Total liabilities |
||||||||||||
Commitments and contingencies |
||||||||||||
Stockholders’ equity: |
||||||||||||
Preferred stock, $ |
||||||||||||
Common stock, $ |
||||||||||||
Additional paid-in capital |
||||||||||||
Accumulated deficit |
( |
) | ( |
) | ( |
) | ||||||
Accumulated other comprehensive loss |
( |
) | ( |
) | ||||||||
Total stockholders’ equity |
||||||||||||
Total liabilities and stockholders’ equity |
$ | $ | $ | |||||||||
As of December 31, 2024 |
||||||||||||
As Reported |
Adjustment |
As Restated |
||||||||||
Assets |
||||||||||||
Current assets: |
||||||||||||
Cash and cash equivalents |
$ | $ | $ | |||||||||
Restricted cash |
||||||||||||
Trade and other receivables |
||||||||||||
Prepaid and other assets |
||||||||||||
Total current assets |
||||||||||||
Property and equipment, net |
||||||||||||
Deposits |
||||||||||||
Prepaid and other assets |
||||||||||||
Right-of-use assets |
||||||||||||
Total assets |
$ | $ | $ | |||||||||
Liabilities and stockholders’ equity |
||||||||||||
Current liabilities: |
||||||||||||
Trade and other payables |
$ | $ | $ | |||||||||
Accrued employee benefits |
||||||||||||
Lease liabilities, current portion |
||||||||||||
Total current liabilities |
||||||||||||
Non-current accrued employee benefit |
||||||||||||
Total liabilities |
||||||||||||
Commitments and contingencies |
||||||||||||
Stockholders’ equity: |
||||||||||||
Preferred stock, $ |
||||||||||||
Common stock, $ |
||||||||||||
Additional paid-in capital |
||||||||||||
Accumulated deficit |
( |
) | ( |
) | ( |
) | ||||||
Accumulated other comprehensive loss |
( |
) | ( |
) | ||||||||
Total stockholders’ equity |
||||||||||||
Total liabilities and stockholders’ equity |
$ | $ | $ | |||||||||
Three Months Ended |
Nine Months Ended |
|||||||||||||||||||||||
March 31, 2025 |
March 31, 2025 |
|||||||||||||||||||||||
As Reported |
Adjustment |
As Restated |
As Reported |
Adjustment |
As Restated |
|||||||||||||||||||
Operating expenses |
||||||||||||||||||||||||
Research and development |
$ | $ | $ | $ | $ | $ | ||||||||||||||||||
General and administrative |
||||||||||||||||||||||||
Total operating expenses |
||||||||||||||||||||||||
Loss from operations |
( |
) | ( |
) | ( |
) | ( |
) | ( |
) | ( |
) | ||||||||||||
Other income (loss): |
||||||||||||||||||||||||
Foreign currency transaction gain (loss) |
( |
) | ( |
) | ||||||||||||||||||||
Interest income (expense), net |
||||||||||||||||||||||||
Other expense, net |
( |
) | ( |
) | ||||||||||||||||||||
Gain on extinguishment of liabilities |
||||||||||||||||||||||||
Total other income, net |
||||||||||||||||||||||||
Net loss |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||||
Other comprehensive income: |
||||||||||||||||||||||||
Unrealized foreign currency translation gain (loss) |
( |
) | ( |
) | ||||||||||||||||||||
Total other comprehensive income (loss) |
( |
) | ( |
) | ||||||||||||||||||||
Total comprehensive loss |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||||
Net loss |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||||
Net loss attributable to common shareholders |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||||
Net loss per share: |
||||||||||||||||||||||||
Basic and diluted |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||||
Weighted average number of shares outstanding: basic and diluted |
||||||||||||||||||||||||
Three Months Ended |
Six Months Ended |
|||||||||||||||||||||||
December 31, 2024 |
December 31, 2024 |
|||||||||||||||||||||||
As Reported |
Adjustment |
As Restated |
As Reported |
Adjustment |
As Restated |
|||||||||||||||||||
Operating expenses |
||||||||||||||||||||||||
Research and development |
$ | $ | $ | $ | $ | $ | ||||||||||||||||||
General and administrative |
||||||||||||||||||||||||
Total operating expenses |
||||||||||||||||||||||||
Loss from operations |
( |
) | ( |
) | ( |
) | ( |
) | ( |
) | ( |
) | ||||||||||||
Other income (loss): |
||||||||||||||||||||||||
Foreign currency transaction gain (loss) |
( |
) | ( |
) | ( |
) | ( |
) | ||||||||||||||||
Interest income (expense), net |
||||||||||||||||||||||||
Other expense, net |
( |
) | ( |
) | ( |
) | ( |
) | ||||||||||||||||
Gain on extinguishment of liabilities |
||||||||||||||||||||||||
Unrealized loss on investment |
||||||||||||||||||||||||
Total other income, net |
||||||||||||||||||||||||
Net loss |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||||
Other comprehensive income: |
||||||||||||||||||||||||
Unrealized foreign currency translation gain (loss) |
||||||||||||||||||||||||
Total other comprehensive income (loss) |
||||||||||||||||||||||||
Total comprehensive loss |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||||
Net loss |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||||
Deemed dividends |
||||||||||||||||||||||||
Net loss attributable to common shareholders |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||||
Net loss per share: |
||||||||||||||||||||||||
Basic and diluted |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||||
Weighted average number of shares outstanding: basic and diluted |
$ | |||||||||||||||||||||||
Common Stock |
Additional Paid-in |
Accumulated |
Accumulated Other Comprehensive |
Total Stockholders’ |
||||||||||||||||||||
Shares |
Amount |
Capital |
Deficit |
Loss |
Equity |
|||||||||||||||||||
Balance at June 30, 2024 |
$ | $ | $ | ( |
) | $ | ( |
) | $ | |||||||||||||||
Exercise of pre-funded warrants |
— | — | — | — | — | |||||||||||||||||||
Exercise of Series 2 warrants |
— | — | — | |||||||||||||||||||||
Exercise of common warrants |
— | — | — | |||||||||||||||||||||
Share-based compensation |
— | — | — | — | ||||||||||||||||||||
Foreign currency translation loss |
— | — | — | — | ( |
) | ( |
) | ||||||||||||||||
Net loss |
— | — | — | ( |
) | — | ( |
) | ||||||||||||||||
Balance at September 30, 2024 |
( |
) | ( |
) | ||||||||||||||||||||
Exercise of pre-funded warrants |
— | — | — | — | — | |||||||||||||||||||
Exercise of Series 2 warrants |
— | — | — | |||||||||||||||||||||
Exercise of common warrants, net of issuance costs of $ |
— | — | — | |||||||||||||||||||||
Share-based compensation |
— | — | — | — | ||||||||||||||||||||
Foreign currency translation gain |
— | — | — | — | ||||||||||||||||||||
Net loss |
— | — | — | ( |
) | — | ( |
) | ||||||||||||||||
Restatement adjustment |
— | — | ( |
) | — | — | ||||||||||||||||||
Balance at December 31, 2024 (as restated) |
$ | $ | $ | ( |
) | $ | ( |
) | $ | |||||||||||||||
Issuance of common stock and pre-funded warrants sold for cash, net of offering costs of $ |
— | — | ||||||||||||||||||||||
Exercise of common warrants |
— | — | — | |||||||||||||||||||||
Share-based compensation |
— | — | — | — | ||||||||||||||||||||
Foreign currency translation gain |
— | — | — | — | ( |
) | ( |
) | ||||||||||||||||
Net loss |
— | — | — | ( |
) | — | ( |
) | ||||||||||||||||
Restatement adjustment |
— | — | ( |
) | — | — | ||||||||||||||||||
Balance at March 31, 2025 (as restated) |
$ | $ | $ | ( |
) | $ | ( |
) | $ | |||||||||||||||
Nine Months Ended March 31, 2025 |
||||||||||||
As Reported |
Adjustment |
As Restated |
||||||||||
Cash flows from operating activities: |
||||||||||||
Net loss |
$ | ( |
) | $ | ( |
) | $ | ( |
) | |||
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
Depreciation and amortization |
||||||||||||
Amortization of right-of-use assets |
||||||||||||
Unrealized loss on investment |
||||||||||||
Gain on extinguishment of liabilities |
( |
) | ( |
) | ||||||||
Share-based compensation expense |
||||||||||||
Changes in operating assets and liabilities: |
||||||||||||
Trade and other receivables |
||||||||||||
Prepaid and other assets |
||||||||||||
Trade and other payables |
||||||||||||
Accrued employee benefits |
( |
) | ( |
) | ||||||||
Lease liabilities |
( |
) | ( |
) | ||||||||
Net cash used in operating activities |
( |
) | ( |
) | ||||||||
Cash flows from investing activities: |
||||||||||||
Purchase of property and equipment |
( |
) | ( |
) | ||||||||
Net cash used in investing activities |
( |
) | ( |
) | ||||||||
Cash flows from financing activities: |
||||||||||||
Proceeds from issuance of common stock, pre-funded warrants, and common warrants |
||||||||||||
Proceeds from exercise of pre-funded warrants, series 2 warrants and common warrants |
||||||||||||
Share issuance transaction costs |
( |
) | ( |
) | ||||||||
Net cash provided by financing activities |
||||||||||||
Effects of exchange rate changes on cash, cash equivalents, and restricted cash |
||||||||||||
Net increase in cash, cash equivalents, and restricted cash |
||||||||||||
Cash, cash equivalents, and restricted cash, beginning of period |
||||||||||||
Cash, cash equivalents, and restricted cash, end of period |
$ | $ | ||||||||||
Supplemental disclosure of cash flow information |
||||||||||||
Initial measurement of operating lease right- of -use assets and liabilities |
$ | $ | ||||||||||
Re-measurement of operating lease right-of-use assets and liabilities |
$ | $ | ||||||||||
Six Months Ended December 31, 2024 |
||||||||||||
As Reported |
Adjustment |
As Restated |
||||||||||
Cash flows from operating activities: |
||||||||||||
Net loss |
$ | ( |
) | $ | ( |
) | $ | ( |
) | |||
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
Depreciation and amortization |
||||||||||||
Amortization of right-of-use assets |
||||||||||||
Unrealized loss on investment |
||||||||||||
Gain on extinguishment of liabilities |
( |
) | ( |
) | ||||||||
Share-based compensation expense |
||||||||||||
Changes in operating assets and liabilities: |
||||||||||||
Trade and other receivables |
||||||||||||
Prepaid and other assets |
||||||||||||
Trade and other payables |
( |
) | ( |
) | ||||||||
Accrued employee benefits |
||||||||||||
Lease liabilities |
( |
) | ( |
) | ||||||||
Net cash used in operating activities |
( |
) | ( |
) | ||||||||
Cash flows from investing activities: |
||||||||||||
Purchase of property and equipment |
( |
) | ( |
) | ||||||||
Net cash used in investing activities |
( |
) | ( |
) | ||||||||
Cash flows from financing activities: |
||||||||||||
Proceeds from exercise of pre-funded warrants, series 2 warrants and common warrants |
||||||||||||
Share issuance transaction costs |
( |
) | ( |
) | ||||||||
Net cash provided by financing activities |
||||||||||||
Effects of exchange rate changes on cash, cash equivalents, and restricted cash |
||||||||||||
Net increase in cash, cash equivalents, and restricted cash |
||||||||||||
Cash, cash equivalents, and restricted cash, beginning of period |
||||||||||||
Cash, cash equivalents, and restricted cash, end of period |
$ | $ | ||||||||||
Three Months Ended |
||||||||||||||||
September 30, 2024 |
December 31, 2024 |
March 31, 2025 |
June 30, 2025 |
|||||||||||||
(Restated) |
(Restated) |
|||||||||||||||
Operating expenses |
||||||||||||||||
Royalties and license fees |
$ | $ | $ | $ | ||||||||||||
Research and development |
||||||||||||||||
General and administrative |
||||||||||||||||
Total operating expenses |
||||||||||||||||
Loss from operations |
( |
) | ( |
) | ( |
) | ( |
) | ||||||||
Other income (loss): |
||||||||||||||||
Foreign currency transaction gain (loss) |
( |
) | ||||||||||||||
Interest income (expense), net |
||||||||||||||||
Other expense, net |
( |
) | ( |
) | ||||||||||||
Gain on extinguishment of liabilities |
||||||||||||||||
Total other income, net |
||||||||||||||||
Net loss |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||
Other comprehensive income: |
||||||||||||||||
Unrealized foreign currency translation gain (loss) |
( |
) | ( |
) | ( |
) | ||||||||||
Total other comprehensive income (loss) |
$ | ( |
) | $ | $ | ( |
) | $ | ( |
) | ||||||
Total comprehensive loss |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||
Net loss |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||
Net loss per share: |
||||||||||||||||
Basic and diluted |
$ |
( |
) |
$ |
( |
) |
$ |
( |
) |
$ |
( |
) | ||||
Net loss per share: |
||||||||||||||||
Weighted average number of shares outstanding: basic and diluted |
||||||||||||||||
| (US$’000) | June 30, 2025 |
June 30, 2024 |
||||||
Cash at bank |
$ | $ | ||||||
Restricted cash |
||||||||
Total |
$ | $ | ||||||
| (US$’000) | June 30, 2025 |
June 30, 2024 |
||||||
Prepaid expenses |
$ | $ | ||||||
Market value of listed shares |
||||||||
Total other assets |
||||||||
Less: non-current portion |
( |
) | ( |
) | ||||
Current portion |
$ | $ | ||||||
| (US$’000) | June 30, 2025 |
June 30, 2024 |
||||||
Software |
$ | $ | ||||||
Lab equipment |
||||||||
Computer hardware |
||||||||
Furniture and fixtures |
||||||||
Leasehold improvements |
||||||||
Total property and equipment, gross |
||||||||
Accumulated depreciation and amortization |
( |
) | ( |
) | ||||
Total property and equipment, net |
$ | $ | ||||||
(US$’000) |
June 30, 2025 |
June 30, 2024 |
||||||
Trade payable |
$ | $ | ||||||
Accrued consultant fees |
||||||||
Accrued professional fees |
||||||||
Accrued clinical development project costs |
||||||||
Other payables |
||||||||
Total |
$ | $ | ||||||
| (US$’000) | Operating lease right- of- use assets |
|||
Balance at July 1, 2024 |
$ | |||
Re-measurement during the period |
||||
Initial measurement at February 1, 2025 |
||||
Amortization of right of use asset |
( |
) | ||
Operating lease right-of-use |
$ | |||
| (US$’000) | Operating lease liabilities |
|||
Balance at July 1, 2024 |
$ | |||
Re-measurement during the period |
||||
Initial measurement at February 1, 2025 |
||||
Principal payments on operating lease liabilities |
( |
) | ||
Operating lease liabilities at June 30 2025 |
||||
Less: non-current portion |
( |
) | ||
Current portion at June 30, 2025 |
$ | |||
| (US$’000) | June 30, 2025 |
|||
2025 |
$ | |||
2026 |
||||
2027 |
||||
Total operating lease payments |
||||
Less imputed interest |
( |
) | ||
Present value of operating lease liabilities |
$ | |||
| June 30, 2025 |
June 30, 2024 |
|||||||
Common stock warrants outstanding |
||||||||
Common stock options issued and outstanding |
||||||||
Shares available for future issuance under the 2020 Plan |
||||||||
Shares reserved for common stock under the At the Market Offering |
||||||||
Total |
||||||||
| Common Stock from Warrants |
Weighted- average Exercise Price (per share) |
|||||||
Outstanding at July 1, 2023 |
$ | |||||||
Pre-funded warrants issued August 11, 2023 |
$ | |||||||
Common warrants issued August 11, 2023 |
$ | |||||||
Pre-funded warrants issued April 22, 2024 |
$ | |||||||
Common warrants exercised |
( |
) | $ | |||||
Series 2 warrants exercised |
( |
) | $ | |||||
Pre-funded warrants exercised |
( |
) | $ | |||||
Outstanding and exercisable at June 30, 2024 |
$ | |||||||
Pre-funded warrants issued March 25, 2025 |
$ | |||||||
Pre-funded warrants exercised |
( |
) | $ | |||||
Series 2 warrants exercised |
( |
) | $ | |||||
Common warrants exercised |
( |
) | $ | |||||
Purchase warrants expired |
( |
) | $ | |||||
Outstanding and exercisable at June 30, 2025 |
$ | |||||||
Stock Options |
Weighted- average Exercise Price |
Weighted- average Remaining Contractual Term |
Aggregate Intrinsic Value |
|||||||||||||
Outstanding at July 1, 2023 |
$ | $ | ||||||||||||||
Granted |
||||||||||||||||
Expired |
( |
) | — | |||||||||||||
Forfeited |
( |
) | — | |||||||||||||
Outstanding at June 30, 2024 |
$ | $ | ||||||||||||||
Granted |
||||||||||||||||
Expired |
— | |||||||||||||||
Forfeited |
— | |||||||||||||||
Outstanding at June 30, 2025 |
$ | $ | ||||||||||||||
Exercisable at June 30, 2025 |
$ | $ | ||||||||||||||
Fiscal Year Ended June 30, |
||||||||||||
2025 |
2024 |
|||||||||||
Expected volatility |
% |
% | ||||||||||
Expected term |
||||||||||||
Risk-free interest rate |
% |
% | ||||||||||
Expected dividend yield |
— |
% |
— |
% | ||||||||
(US$’000) |
June 30, |
|||||||
2025 |
2024 |
|||||||
Research and development |
$ | $ | ||||||
General and administrative |
||||||||
Total share-based compensation expense |
$ | $ | ||||||
(US$’000) |
Year Ended June 30, |
|||||||
2025 |
2024 |
|||||||
United States |
$ | ( |
) | $ | ( |
) | ||
International |
( |
) | ( |
) | ||||
Total |
$ | ( |
) | $ | ( |
) | ||
(US$’000) |
June 30, |
|||||||
2025 |
2024 |
|||||||
Deferred tax assets: |
||||||||
Net operating losses |
$ |
$ |
||||||
Other |
||||||||
Lease liability |
||||||||
Share-based compensation |
||||||||
Intangible assets |
||||||||
Section 174 Capitalization |
||||||||
Gross deferred tax assets |
||||||||
Less valuation allowance |
( |
) |
( |
) | ||||
Deferred tax liabilities: |
||||||||
Right-of-use |
( |
) |
( |
) | ||||
Fixed assets |
( |
) |
( |
) | ||||
Prepaid expenses |
( |
) |
( |
) | ||||
Unrealized foreign exchange gains and losses |
( |
) |
( |
) | ||||
Total deferred tax liabilities |
( |
) |
( |
) | ||||
Net deferred taxes |
$ |
$ |
||||||
(US$’000) |
Amount |
Expiration Years |
||||||
| Net operating losses, federal (post-December 31, 2017) |
$ | |||||||
| Net operating losses, state |
||||||||
| Net operating losses, Australia |
||||||||
Year Ended June 30, |
||||||||
2025 |
2024 |
|||||||
| Statutory rate |
% |
% | ||||||
| Permanent differences |
( |
%) | ( |
%) | ||||
| Share-based payments |
( |
%) |
( |
%) | ||||
| Change in valuation allowance |
( |
%) | % | |||||
| Foreign tax rate differential |
% |
% | ||||||
| Section 382 Write-off |
( |
%) |
( |
%) | ||||
| Section 162m Write-off |
|
|
( |
%) |
|
|
|
% |
| |
|
|
|
|||||
| Total |
( |
%) |
( |
%) | ||||
| |
|
|
|
|||||
(US$’000) |
Fiscal Year Ended June 30, |
|||||||
Operating Expenses |
2025 |
2024 |
||||||
Royalties and license fees |
$ |
$ |
( |
) | ||||
Research and development |
||||||||
General and administrative |
||||||||
Other segment items |
( |
) |
( |
) | ||||
Net loss |
( |
) |
( |
) | ||||
Table of Contents
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and principal financial officer, evaluated the effectiveness of our disclosure controls and procedures pursuant to Rule 13a-15 under the Securities Exchange Act of 1934, as of June 30, 2025. In designing and evaluating our disclosure controls and procedures, our management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives, and our management necessarily applied its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on this evaluation, our principal executive officer and principal financial officer concluded that, have concluded that our disclosure controls and procedures were not effective as of June 30, 2025 because of the material weakness identified in our internal control over financial reporting.
Changes in Internal Control Over Financial Reporting
Our management, including our principal executive and principal financial officer, has evaluated any changes in our internal control over financial reporting that occurred during the year ended June 30, 2025, and noting the material weakness, discussed below, has otherwise concluded that there was no change that occurred during that period that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rule 13a-15(f) under the Exchange Act. Our management, with the participation of our principal executive and principal financial officer, conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, our management has concluded that our internal control over financial reporting was not effective as of June 30, 2025 due to a material weakness in our internal controls as described below.
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis.
In connection with our audited consolidated financial statements for the year ended June 30, 2025, management identified a material weakness in our internal control over financial reporting with respect to:
| • | Inadequate design and implementation of controls over our share-based compensation calculation review process. Specifically, we did not design and/or implement process level controls to ensure all inputs used in share-based compensation expense calculations are complete and accurate, including review of the vesting allocation method applied by the equity system. |
The material weakness resulted in the restatement of the consolidated financial statements for the quarterly periods as of and for the periods ended March 31, 2025, and December 31, 2024.
81
Table of Contents
Notwithstanding the material weakness identified above, management has concluded that our consolidated financial statements included in this Annual Report fairly present in all material respects the financial condition, results of operations and cash flows of the Company in accordance with US GAAP for each of the periods presented therein.
In order to remediate this matter, we plan to perform, or already have performed, the following remediating steps:
| • | Updated the equity system’s default vesting allocation method configuration; |
| • | Enhanced management’s quarterly share-based compensation review process to identify and verify all relevant inputs of the share-based compensation expense calculation, including review over the completeness and accuracy of the vesting allocation method applied by the equity system. |
We will consider the material weakness to be fully remediated once the applicable controls operate for a sufficient period of time and our management has concluded, through testing, that these controls are operating effectively.
Remediation of Previously Reported Material Weakness in Internal Control Over Financial Reporting
As reported in Part II, Item 9A, Controls and Procedures, of our Annual Reports on Form 10-K for the fiscal year ended June 30, 2024, filed on September 26, 2024, we previously identified a material weakness in our internal controls resulting from our accounting personnel not being able to process and account for complex, non-routine transactions in accordance with US GAAP.
Since identifying the material weakness, our management has designed and implemented new or enhanced internal control procedures, which we believe address both the identified material weakness and strengthens our overall financial control environment, including:
| • | Adding financial reporting controls over the review of significant and unusual transactions, review of the quarterly disclosure checklist and review of EPS computations; |
| • | Formalizing the flow of business transactions communicated to the accounting function; |
| • | Hiring additional accounting personnel to allow for more robust review of complex, non-routine transactions to prevent similar occurrences in the future; |
| • | Engaging, as necessary, an accounting advisory firm with technical accounting expertise to assist in the accounting and reporting of complex, non-routine transactions; and |
| • | Increasing access to accounting literature and research materials, including participation in ongoing continuing education requirements and periodically hosted accounting and reporting conferences. |
Our management has completed the implementation of significant enhancements to our procedures and newly implemented controls. Based on the results of our testing, our management has determined that the newly implemented controls have been designed and operating effectively for a sufficient period to conclude that the previously identified material weakness was remediated as of June 30, 2025.
Limitations on Effectiveness of Controls and Procedures
In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply its judgment in evaluating the benefits of possible controls and procedures relative to their costs.
We are a non-accelerated filer, and therefore our independent registered public accounting firm has not and is not required to issue a report on the effectiveness of internal control over financial reporting.
82
Table of Contents
Table of Contents
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
The information required by this item with respect to our directors and executive officers will be contained in the Proxy Statement under the caption “Our Management” and is incorporated herein by reference. The information required by this item with respect to our corporate governance will be contained in the Proxy Statement under the caption “Corporate Governance and Board Meetings and Committees” and is incorporated herein by reference.
If required, the information regarding compliance with Section 16(a) of the Exchange Act is to be included in the section entitled “Delinquent Section 16(a) Reports.”
We have adopted a written Code of Ethics and Business Conduct (“Code of Conduct”) that applies to all officers, directors and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. The Code of Conduct is available on our website at www.benitec.com. If we make any substantive amendments to the Code of Conduct or grant any waiver from a provision of the Code of Conduct to any executive officer or director, we will promptly disclose the nature of the amendment or waiver on our website in lieu of filing such waiver or amendment in a Current Report on Form 8-K.
Item 11. Executive Compensation.
The information required by this item with respect to our compensation of our directors will be contained in the Proxy Statement under the caption “Director Compensation” and is incorporated herein by reference. The information required by this item with respect to our compensation of our executive officers will be contained in the Proxy Statement under the caption “Executive Compensation” and is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The information required by this item will be contained in the Proxy Statement under the caption “Voting Securities of Principal Stockholders and Management” and is incorporated herein by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
The information required by this item will be contained in the Proxy Statement under the caption “Certain Relationships and Related Party Transactions” and “Corporate Governance and Board meetings and Committees” and is incorporated herein by reference.
Item 14. Principal Accountant Fees and Services.
The information required by this item will be contained in the Proxy Statement under the caption “Ratification of Appointment of Independent Registered Public Accounting Firm” and is incorporated herein by reference.
III-1
Table of Contents
PART IV
Item 15. Exhibits and Financial Statement Schedules.
Financial Statements
Reference is made to the Index to the Consolidated Financial Statements included in Item 8 of this report.
Financial Statement Schedules
Required information is included in the notes to the consolidated financial statements.
Exhibit Index
IV-1
Table of Contents
IV-2
Table of Contents
IV-3
Table of Contents
| Exhibit Number |
Exhibit | |
| 23.1* | Consent of Baker Tilly US, LLP | |
| 31.1* | Certification of Principal Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 31.2* | Certification of Principal Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 32.1* | Certification of Principal Executive Officer Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 32.2* | Certification of Principal Financial Officer Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 97* | Benitec Biopharma Inc. Dodd-Frank Clawback Policy | |
| 101.INS | Instance Document | |
| 101.SCH | Inline XBRL Taxonomy Extension Schema Document | |
| 101.CAL | Inline XBRL Taxonomy Extension Calculation Linkbase Document | |
| 101.DEF | Inline XBRL Taxonomy Extension Definition Linkbase Document | |
| 101.LAB | Inline XBRL Taxonomy Extension Label Linkbase Document | |
| 101.PRE | Inline XBRL Taxonomy Extension Presentation Linkbase Document | |
| 104 | Cover Page Interactive Data File – the cover page interactive data file does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. | |
| † | Indicates a management contract or compensatory plan. |
| * | Filed or furnished herewith |
Financial Statements and Schedules of Subsidiaries and Affiliates
None.
Item 16. Form 10-K Summary.
Not applicable.
IV-4
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized, in the city of Hayward, State of California, on the 22nd day of September 2025.
| BENITEC BIOPHARMA INC. | ||
| By: | /s/ Dr. Jerel Banks | |
| Dr. Jerel Banks | ||
| Chief Executive Officer | ||
POWER OF ATTORNEY
BY THESE PRESENTS, each person whose signature appears below constitutes and appoints Dr. Jerel Banks and Megan Boston his true and lawful attorney-in-fact and agents, with full power of substitution and resubstitution for him and in his name, place and stead, in any and all capacities to sign any and all amendments to this annual report on Form 10-K, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the U.S. Securities and Exchange Commission, hereby ratifying and confirming all that said attorney-in-fact or his substitute, each acting alone, may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ Dr. Jerel Banks Dr. Jerel Banks |
Chief Executive Officer, Chairman of the Board and Director (principal executive officer) | September 22, 2025 | ||
| /s/ Megan Boston Megan Boston |
Chief Financial Officer, Director (principal accounting and financial |
September 22, 2025 | ||
| /s/ J. Kevin Buchi J. Kevin Buchi |
Director | September 22, 2025 | ||
| /s/ Peter Francis Peter Francis |
Director | September 22, 2025 | ||
| /s/ Edward Smith Edward Smith |
Director | September 22, 2025 | ||
| /s/ Kishan (“Kishen”) Mehta Kishan (“Kishen”) Mehta |
Director | September 22, 2025 | ||
IV-5