EX-99.3
Published on November 3, 2025

Positive Clinical Data Supporting Advancement of BB-301 in OPMD WEBCAST: November 3, 2025 ©2025 Benitec Biopharma. All rights reserved. OCULOPHARYNGEAL MUSCULAR DYSTROPHY (OPMD) A Debilitating, Progressive Disease With No Approved Therapies NASDAQ BNTC
Safe Harbor Statement Except for the historical information set forth herein, the matters set forth in this presentation include forward-looking statements, including statements regarding Benitec’s plans to develop and commercialize its product candidates, the timing of the completion of pre-clinical and clinical trials, the timing of the availability of data from our clinical trials, the timing and sufficiency of patient enrollment and dosing in clinical trials, the timing of expected regulatory filings, and potential clinical utility and potential attributes and benefits of ddRNAi and Benitec’s product candidates, and other forward-looking statements. These forward-looking statements are based on the Company’s current expectations and patient to risks and uncertainties that may cause actual results to differ materially, including unanticipated developments in and risks related to: the success of our plans to develop and potentially commercialize our product candidates; the timing of the completion of preclinical studies and clinical trials; the timing and sufficiency of patient enrollment and dosing in any future clinical trials; the timing of the availability of data from our clinical trials; the timing and outcome of regulatory filings and approvals; the development of novel AAV vectors; our potential future out-licenses and collaborations; the plans of licensees of our technology; the clinical utility and potential attributes and benefits of ddRNAi and our product candidates, including the potential duration of treatment effects and the potential for a “one shot” cure; our intellectual property position and the duration of our patent portfolio; expenses, ongoing losses, future revenue, capital needs and needs for additional financing, and our ability to access additional financing given market conditions and other factors; the length of time over which we expect our cash and cash equivalents to be sufficient to execute on our business plan; unanticipated delays; further research and development and the results of clinical trials possibly being unsuccessful or insufficient to meet applicable regulatory standards or warrant continued development; the ability to enroll sufficient numbers of patients in clinical trials; determinations made by the FDA and other governmental authorities and other regulatory developments; the Company’s ability to protect and enforce its patents and other intellectual property rights; the Company’s dependence on its relationships with its collaboration partners and other third parties; the efficacy or safety of the Company’s products and the products of the Company’s collaboration partners; the acceptance of the Company’s products and the products of the Company’s collaboration partners in the marketplace; market competition; sales, marketing, manufacturing and distribution requirements; greater than expected expenses; expenses relating to litigation or strategic activities; the impact of, and our ability to remediate, the identified material weakness in our internal controls over financial reporting; the impact of local, regional, and national and international economic conditions and events; and other risks detailed from time to time in the Company’s reports filed with the Securities and Exchange Commission. The Company disclaims any intent or obligation to update these forward-looking statements. November 3, 2025 BB-301 Clinical Data
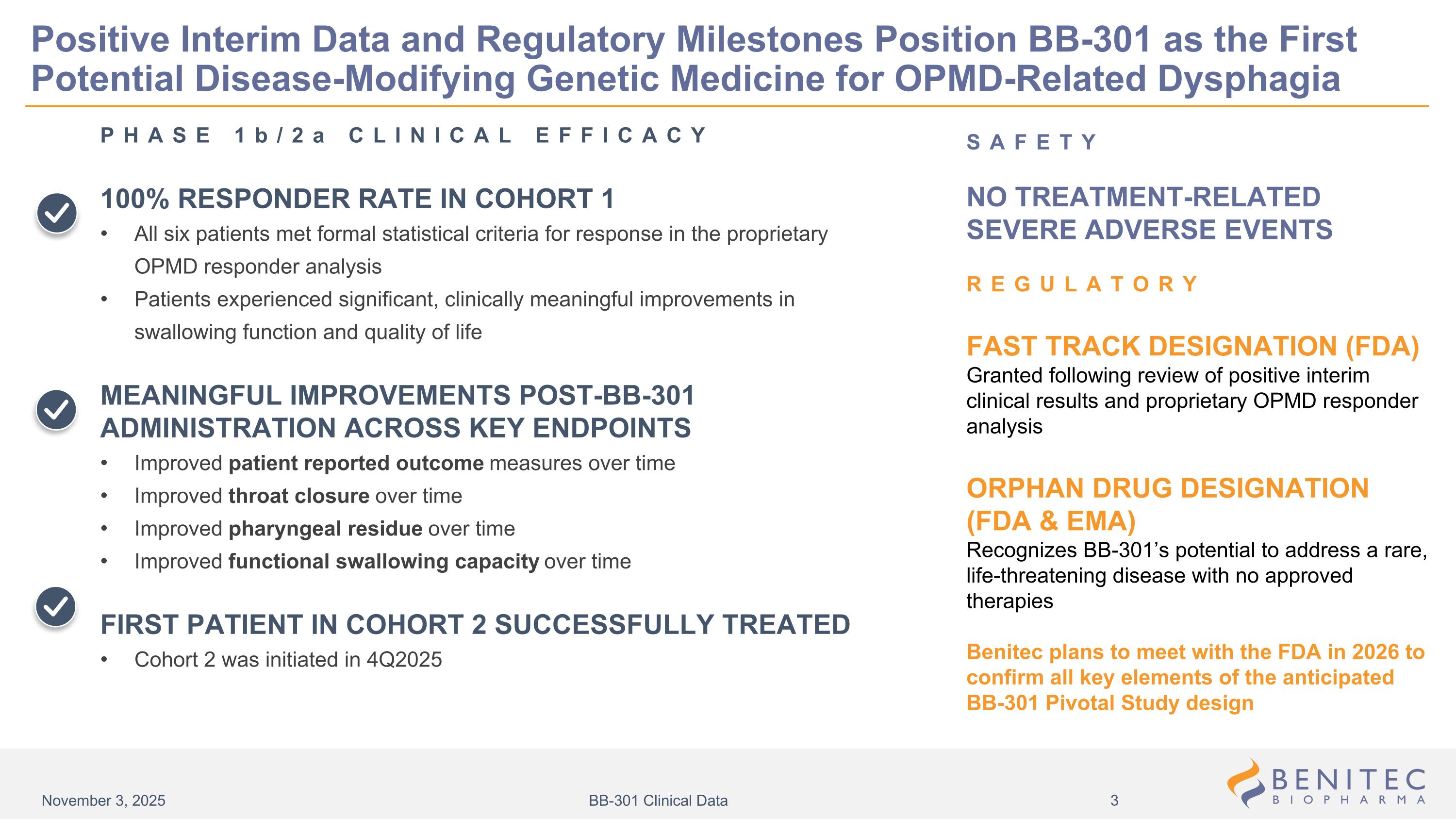
SAFETY NO TREATMENT-RELATED SEVERE ADVERSE EVENTS REGULATORY FAST TRACK DESIGNATION (FDA) Granted following review of positive interim clinical results and proprietary OPMD responder analysis ORPHAN DRUG DESIGNATION (FDA & EMA) Recognizes BB-301’s potential to address a rare, life-threatening disease with no approved therapies Benitec plans to meet with the FDA in 2026 to confirm all key elements of the anticipated BB-301 Pivotal Study design Positive Interim Data and Regulatory Milestones Position BB-301 as the First Potential Disease-Modifying Genetic Medicine for OPMD-Related Dysphagia PHASE 1b/2a CLINICAL EFFICACY 100% RESPONDER RATE IN COHORT 1 All six patients met formal statistical criteria for response in the proprietary OPMD responder analysis Patients experienced significant, clinically meaningful improvements in swallowing function and quality of life MEANINGFUL IMPROVEMENTS POST-BB-301 ADMINISTRATION ACROSS KEY ENDPOINTS Improved patient reported outcome measures over time Improved throat closure over time Improved pharyngeal residue over time Improved functional swallowing capacity over time FIRST PATIENT IN COHORT 2 SUCCESSFULLY TREATED Cohort 2 was initiated in 4Q2025 November 3, 2025 BB-301 Clinical Data

Oculopharyngeal Muscular Dystrophy BB-301: A Novel Disease-Modifying Genetic Medicine
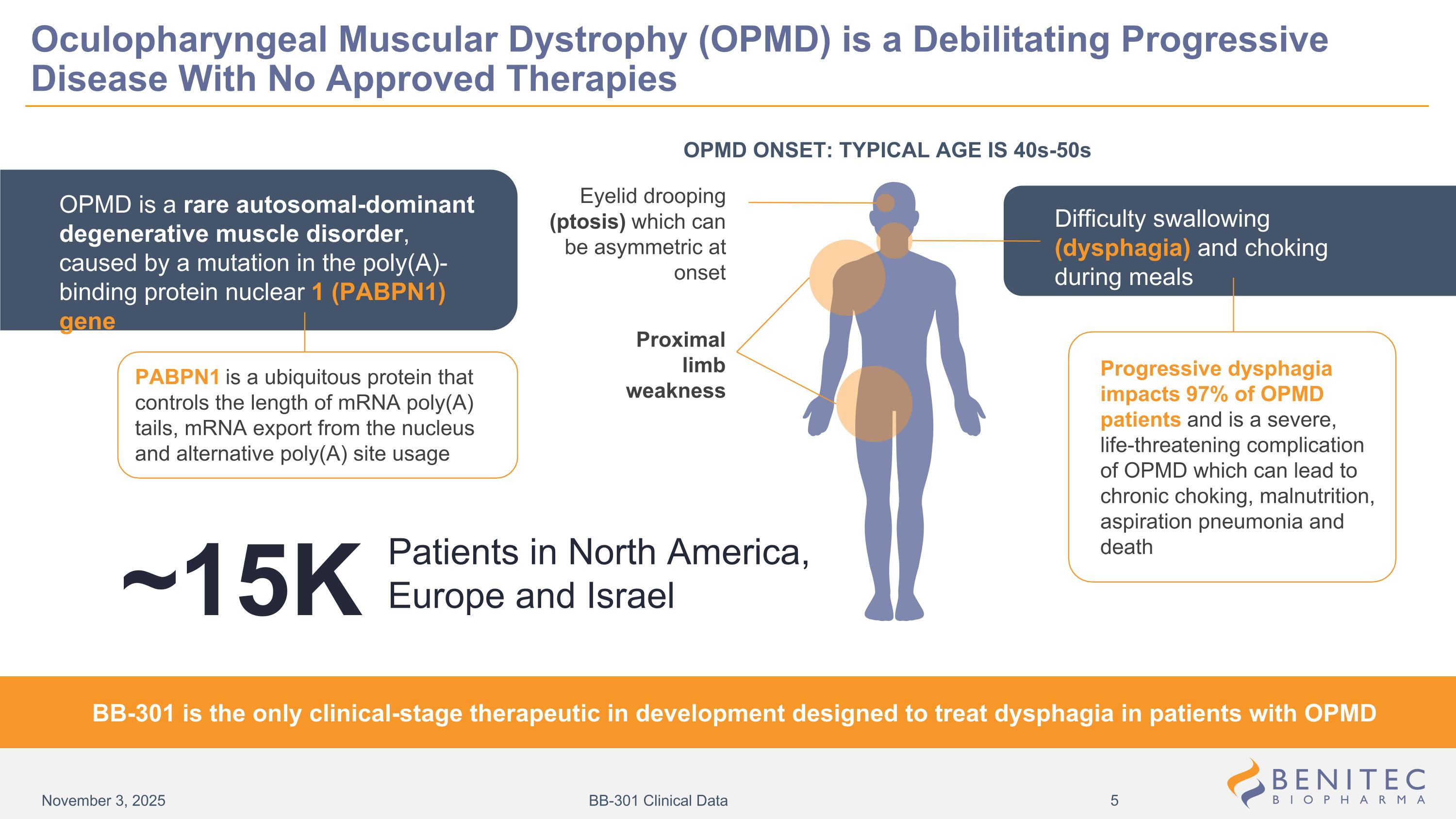
Oculopharyngeal Muscular Dystrophy (OPMD) is a Debilitating Progressive Disease With No Approved Therapies OPMD ONSET: TYPICAL AGE IS 40s-50s Eyelid drooping (ptosis) which can be asymmetric at onset Difficulty swallowing (dysphagia) and choking during meals Proximal limb weakness PABPN1 is a ubiquitous protein that controls the length of mRNA poly(A) tails, mRNA export from the nucleus and alternative poly(A) site usage Progressive dysphagia impacts 97% of OPMD patients and is a severe, life-threatening complication of OPMD which can lead to chronic choking, malnutrition, aspiration pneumonia and death BB-301 is the only clinical-stage therapeutic in development designed to treat dysphagia in patients with OPMD OPMD is a rare autosomal-dominant degenerative muscle disorder, caused by a mutation in the poly(A)-binding protein nuclear 1 (PABPN1) gene Patients in North America, Europe and Israel ~15K November 3, 2025 BB-301 Clinical Data
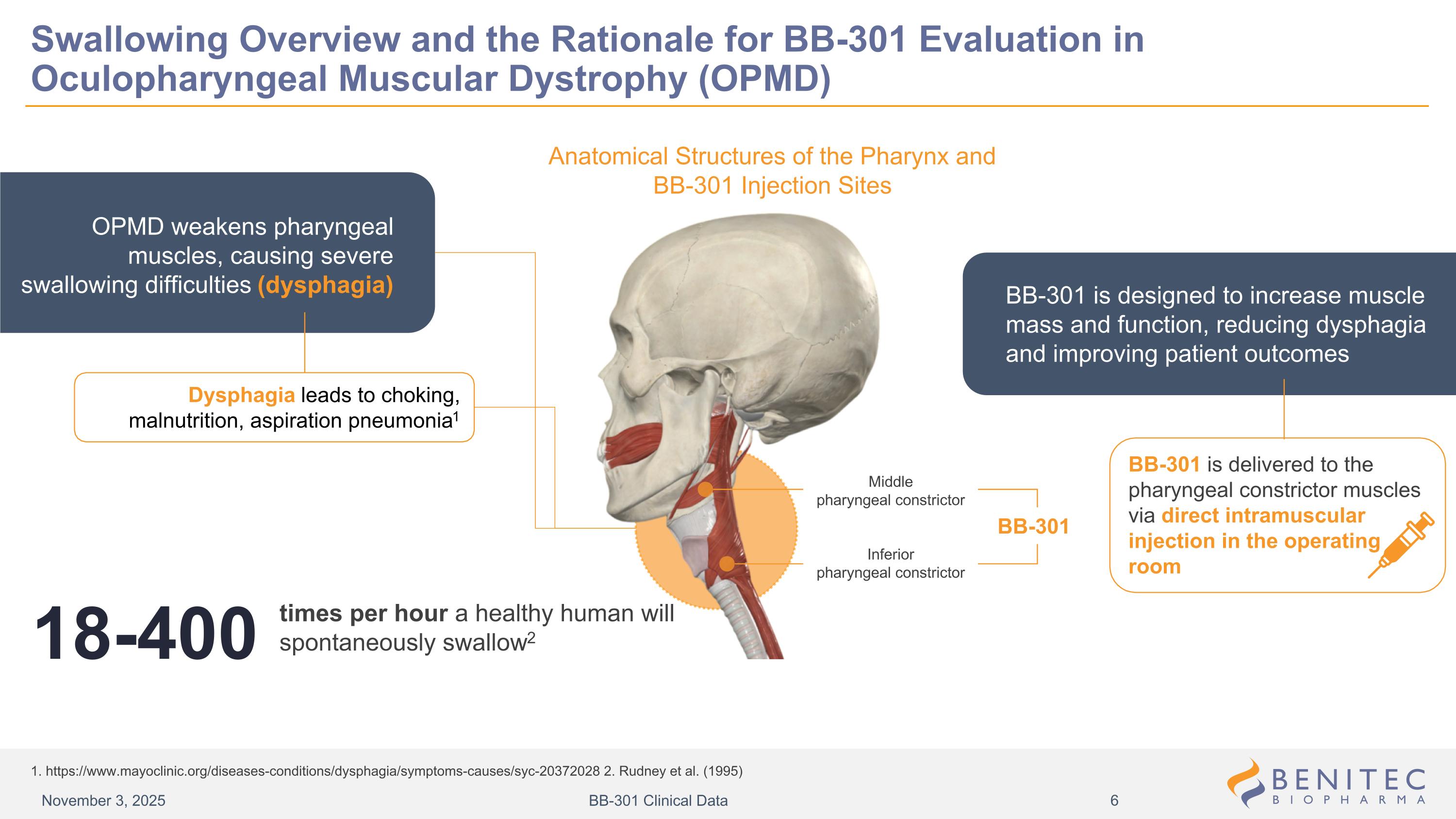
Swallowing Overview and the Rationale for BB-301 Evaluation in Oculopharyngeal Muscular Dystrophy (OPMD) 1. https://www.mayoclinic.org/diseases-conditions/dysphagia/symptoms-causes/syc-20372028 2. Rudney et al. (1995) Anatomical Structures of the Pharynx and BB-301 Injection Sites BB-301 Middle pharyngeal constrictor Inferior pharyngeal constrictor OPMD weakens pharyngeal muscles, causing severe swallowing difficulties (dysphagia) Dysphagia leads to choking, malnutrition, aspiration pneumonia1 times per hour a healthy human will spontaneously swallow2 18-400 BB-301 is designed to increase muscle mass and function, reducing dysphagia and improving patient outcomes BB-301 is delivered to the pharyngeal constrictor muscles via direct intramuscular injection in the operating room November 3, 2025 BB-301 Clinical Data
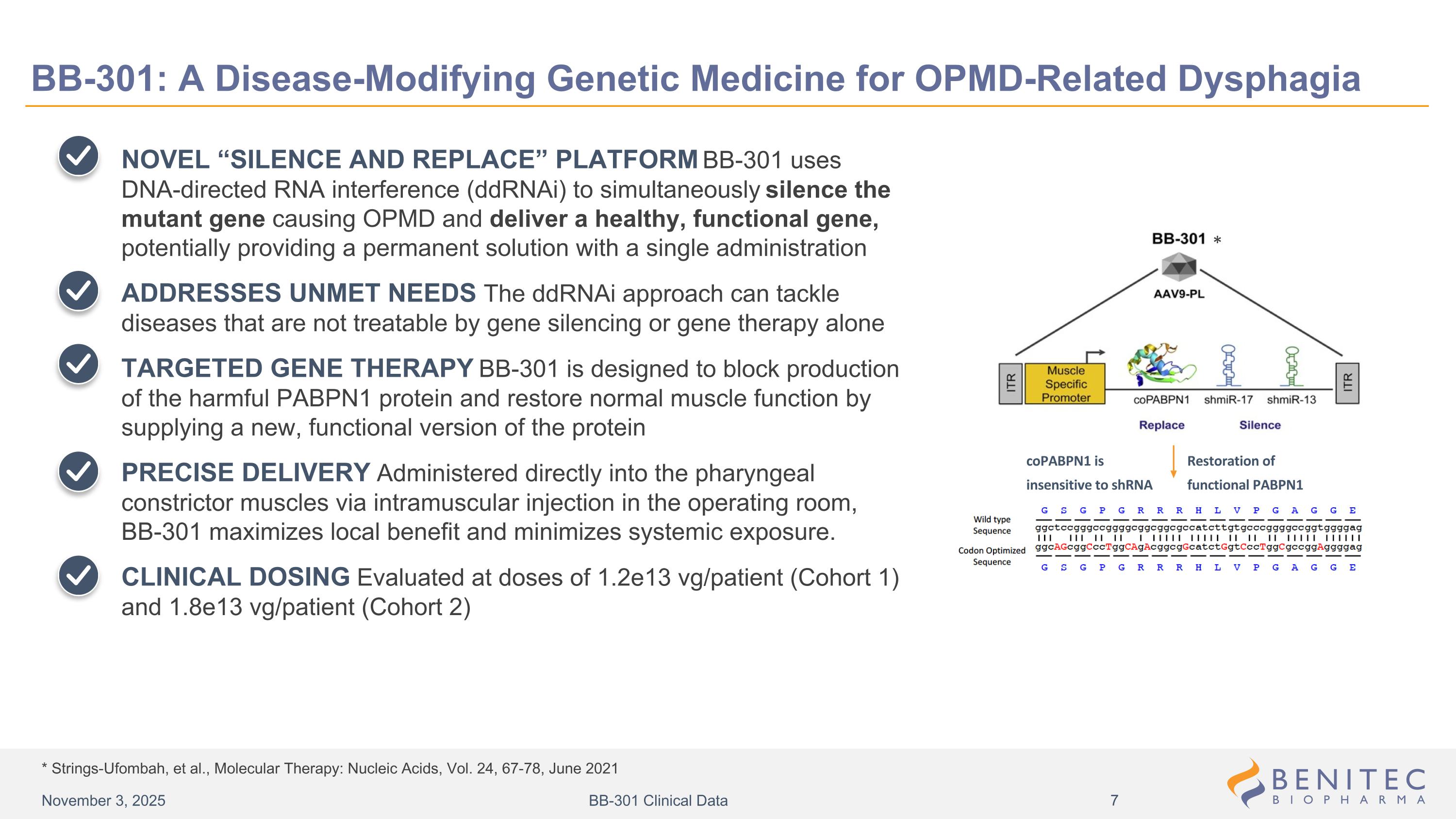
* Strings-Ufombah, et al., Molecular Therapy: Nucleic Acids, Vol. 24, 67-78, June 2021 BB-301: A Disease-Modifying Genetic Medicine for OPMD-Related Dysphagia NOVEL “SILENCE AND REPLACE” PLATFORM BB-301 uses DNA-directed RNA interference (ddRNAi) to simultaneously silence the mutant gene causing OPMD and deliver a healthy, functional gene, potentially providing a permanent solution with a single administration ADDRESSES UNMET NEEDS The ddRNAi approach can tackle diseases that are not treatable by gene silencing or gene therapy alone TARGETED GENE THERAPY BB-301 is designed to block production of the harmful PABPN1 protein and restore normal muscle function by supplying a new, functional version of the protein PRECISE DELIVERY Administered directly into the pharyngeal constrictor muscles via intramuscular injection in the operating room, BB-301 maximizes local benefit and minimizes systemic exposure. CLINICAL DOSING Evaluated at doses of 1.2e13 vg/patient (Cohort 1) and 1.8e13 vg/patient (Cohort 2) Design of Lead Candidate BB-301 Restoration of functional PABPN1 * coPABPN1 is insensitive to shRNA November 3, 2025 BB-301 Clinical Data

Study Design and Key Efficacy Endpoints of the OPMD Natural History Study and BB-301 Phase 1b/2a Clinical Study
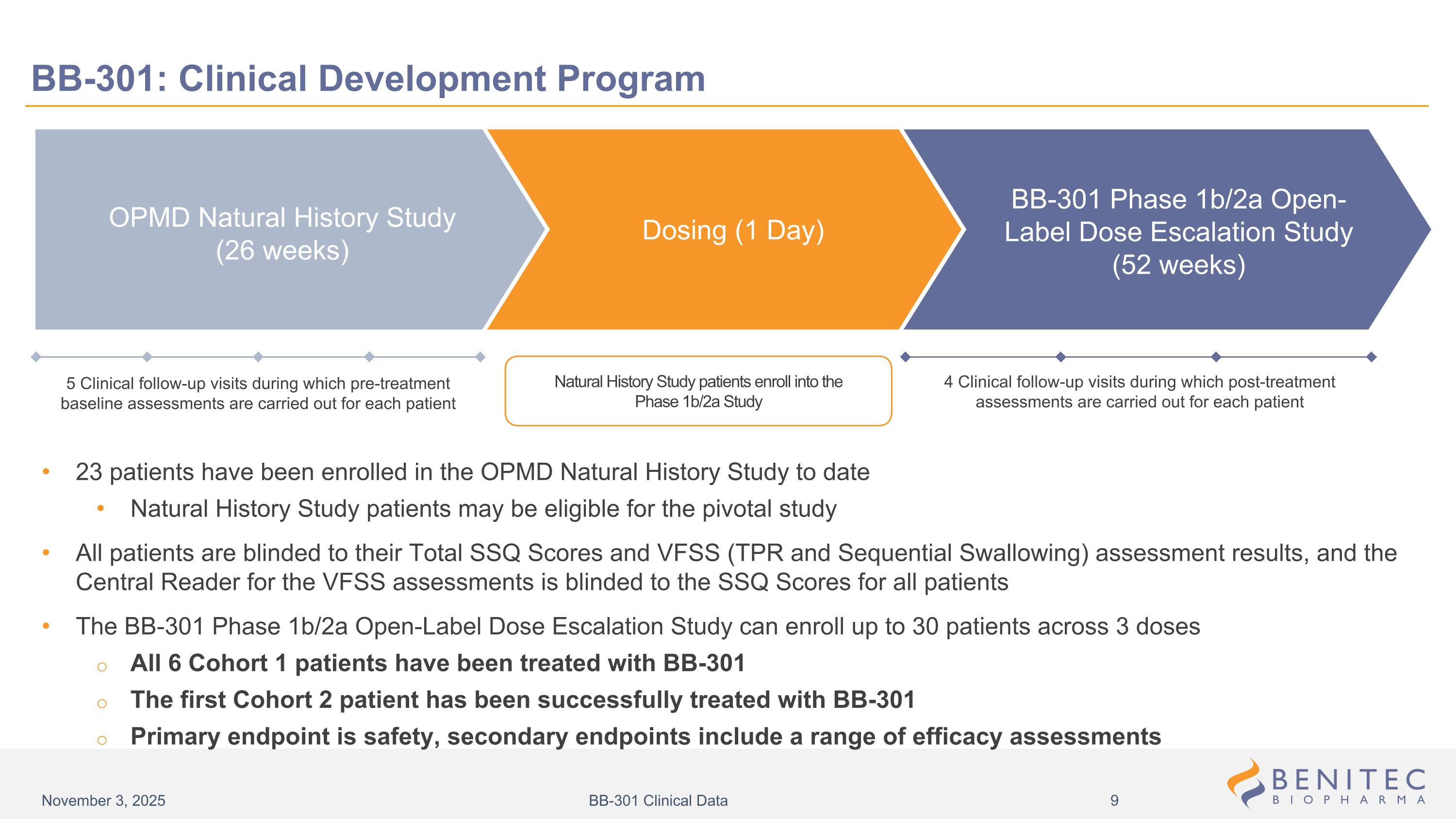
5 Clinical follow-up visits during which pre-treatment baseline assessments are carried out for each patient Natural History Study patients enroll into the Phase 1b/2a Study 4 Clinical follow-up visits during which post-treatment assessments are carried out for each patient BB-301: Clinical Development Program 23 patients have been enrolled in the OPMD Natural History Study to date Natural History Study patients may be eligible for the pivotal study All patients are blinded to their Total SSQ Scores and VFSS (TPR and Sequential Swallowing) assessment results, and the Central Reader for the VFSS assessments is blinded to the SSQ Scores for all patients The BB-301 Phase 1b/2a Open-Label Dose Escalation Study can enroll up to 30 patients across 3 doses All 6 Cohort 1 patients have been treated with BB-301 The first Cohort 2 patient has been successfully treated with BB-301 Primary endpoint is safety, secondary endpoints include a range of efficacy assessments BB-301 Phase 1b/2a Open-Label Dose Escalation Study (52 weeks) Dosing (1 Day) OPMD Natural History Study (26 weeks) November 3, 2025 BB-301 Clinical Data
The Natural History Study and BB-301 Phase 1b/2a Clinical Study include comprehensive assessments of dysphagia approximately every 3 months The total dysphagic symptom burden experienced by OPMD patients has several known underlying contributors The serial assessments of dysphagia facilitated the creation of a multi-component responder analysis which incorporates multiple discrete assessments that holistically assess disease progression and treatment benefit of BB-301 Patient-Reported Oral-Pharyngeal Dysphagia via use of a clinically validated 17-question patient reported outcome instrument, Sydney Swallow Questionnaire (SSQ) BB-301: Serial Characterization of Dysphagia Severity Informs Efficacy Assessment Efficacy assessments were derived from literature-based methods used to assess OPMD dysphagic symptom burden and include patient-reported outcomes, objective anatomic assessments, and functional parameters Videofluoroscopic Swallowing Studies (VFSS) of liquids and solids for anatomic and functional assessments of: Pharyngeal constrictor muscle function via assessment of Pharyngeal Area at Maximum Pharyngeal Constriction (PhAMPC) Swallowing Efficiency via assessment of post-swallow accumulation of food/liquid material Total Pharyngeal Residue (TPR) Normalized Residue Ratio Scale, Valleculae (NRRSv) Swallowing Effectiveness via assessment of frequency of pathologic sequential swallows (SEQ) Functional swallowing capacity as measured by the Cold Water Timed Drinking Test (CWDT) November 3, 2025 BB-301 Clinical Data
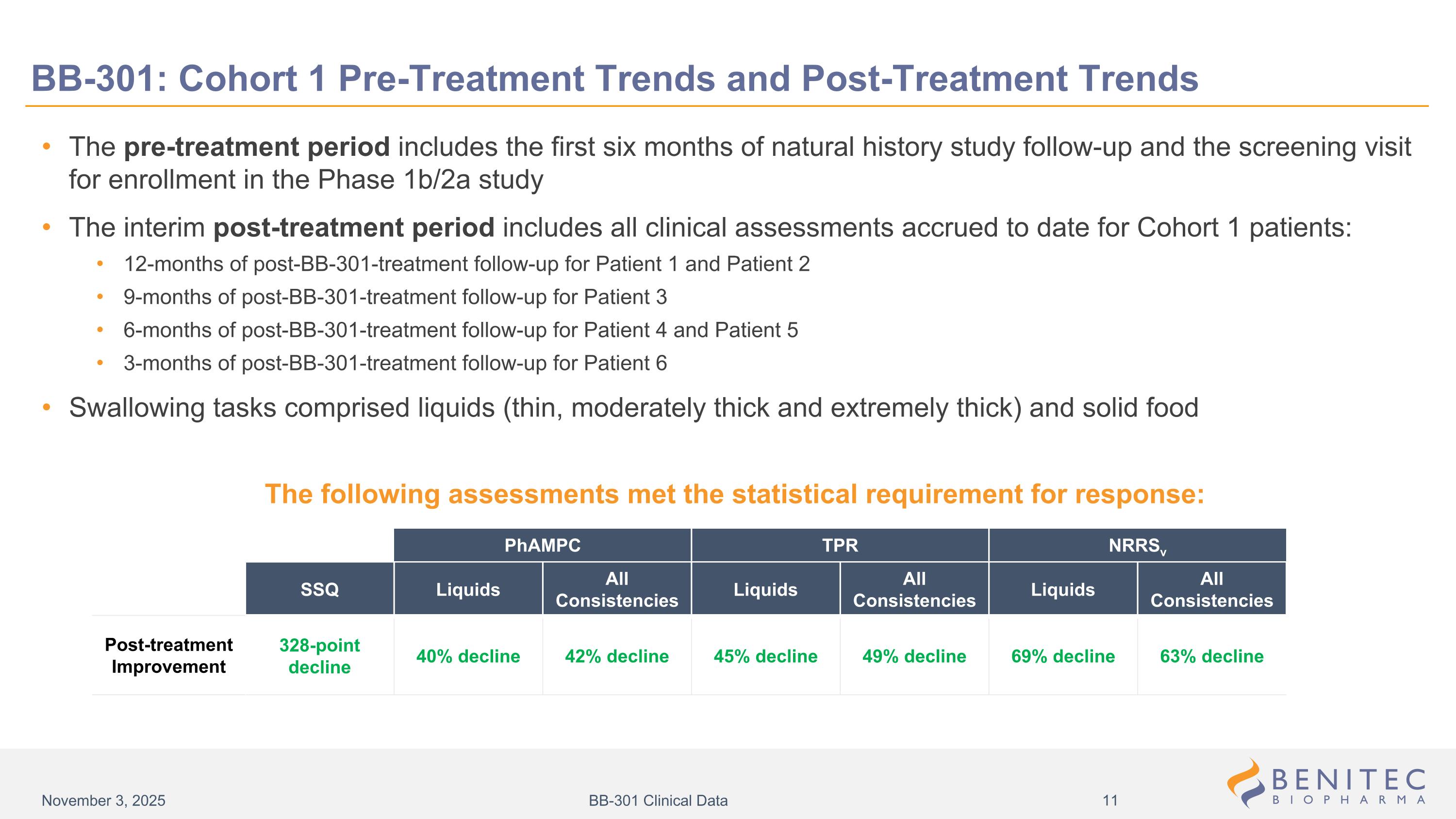
BB-301: Cohort 1 Pre-Treatment Trends and Post-Treatment Trends The pre-treatment period includes the first six months of natural history study follow-up and the screening visit for enrollment in the Phase 1b/2a study The interim post-treatment period includes all clinical assessments accrued to date for Cohort 1 patients: 12-months of post-BB-301-treatment follow-up for Patient 1 and Patient 2 9-months of post-BB-301-treatment follow-up for Patient 3 6-months of post-BB-301-treatment follow-up for Patient 4 and Patient 5 3-months of post-BB-301-treatment follow-up for Patient 6 Swallowing tasks comprised liquids (thin, moderately thick and extremely thick) and solid food SSQ Liquids All Consistencies Liquids All Consistencies Liquids All Consistencies 328-point decline 40% decline 42% decline 45% decline 49% decline 69% decline 63% decline PhAMPC TPR NRRSv Post-treatment Improvement The following assessments met the statistical requirement for response: November 3, 2025 BB-301 Clinical Data
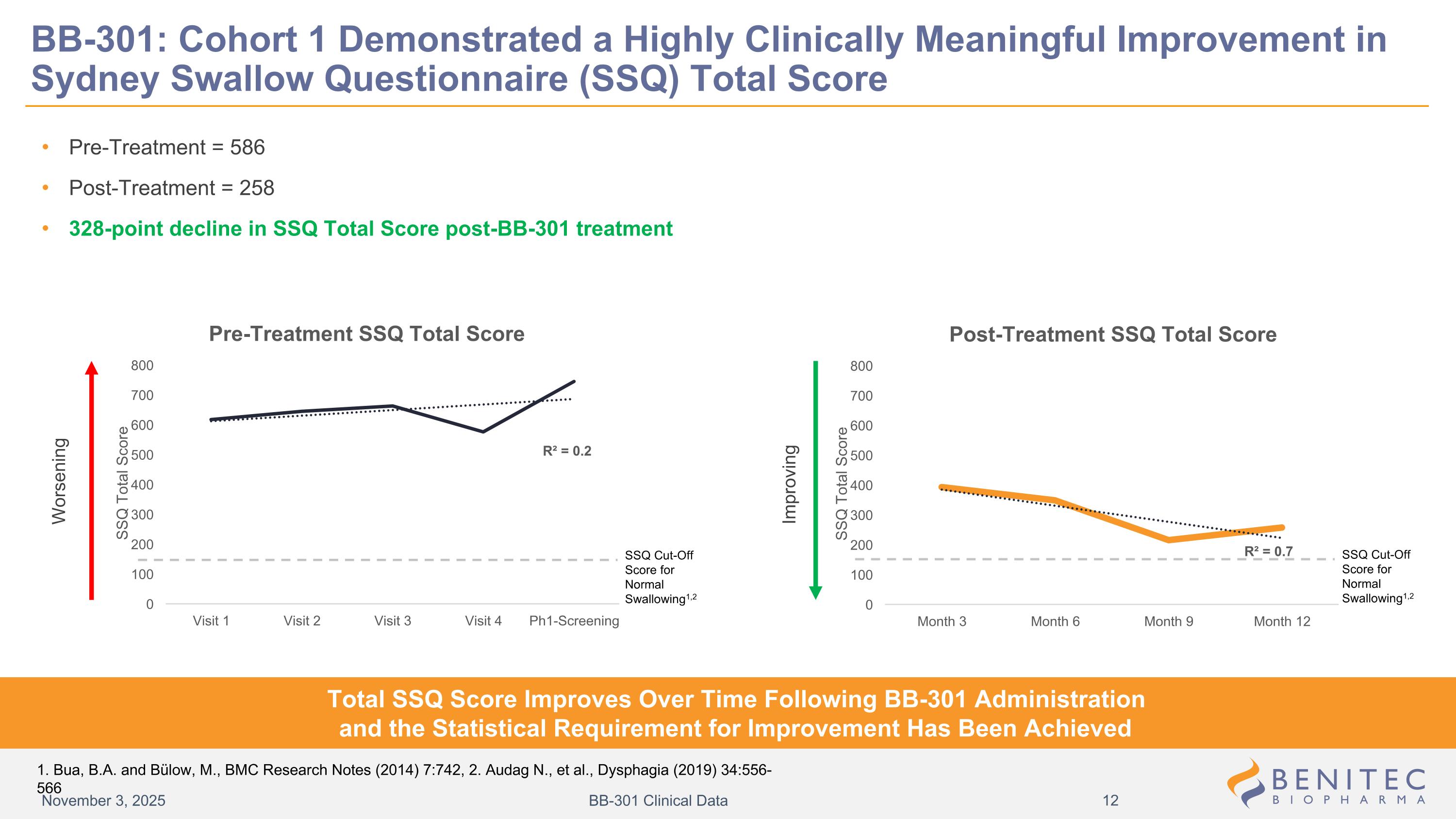
BB-301: Cohort 1 Demonstrated a Highly Clinically Meaningful Improvement in Sydney Swallow Questionnaire (SSQ) Total Score Pre-Treatment = 586 Post-Treatment = 258 328-point decline in SSQ Total Score post-BB-301 treatment Worsening Improving Total SSQ Score Improves Over Time Following BB-301 Administration and the Statistical Requirement for Improvement Has Been Achieved November 3, 2025 BB-301 Clinical Data SSQ Cut-Off Score for Normal Swallowing1,2 SSQ Cut-Off Score for Normal Swallowing1,2 1. Bua, B.A. and Bülow, M., BMC Research Notes (2014) 7:742, 2. Audag N., et al., Dysphagia (2019) 34:556-566
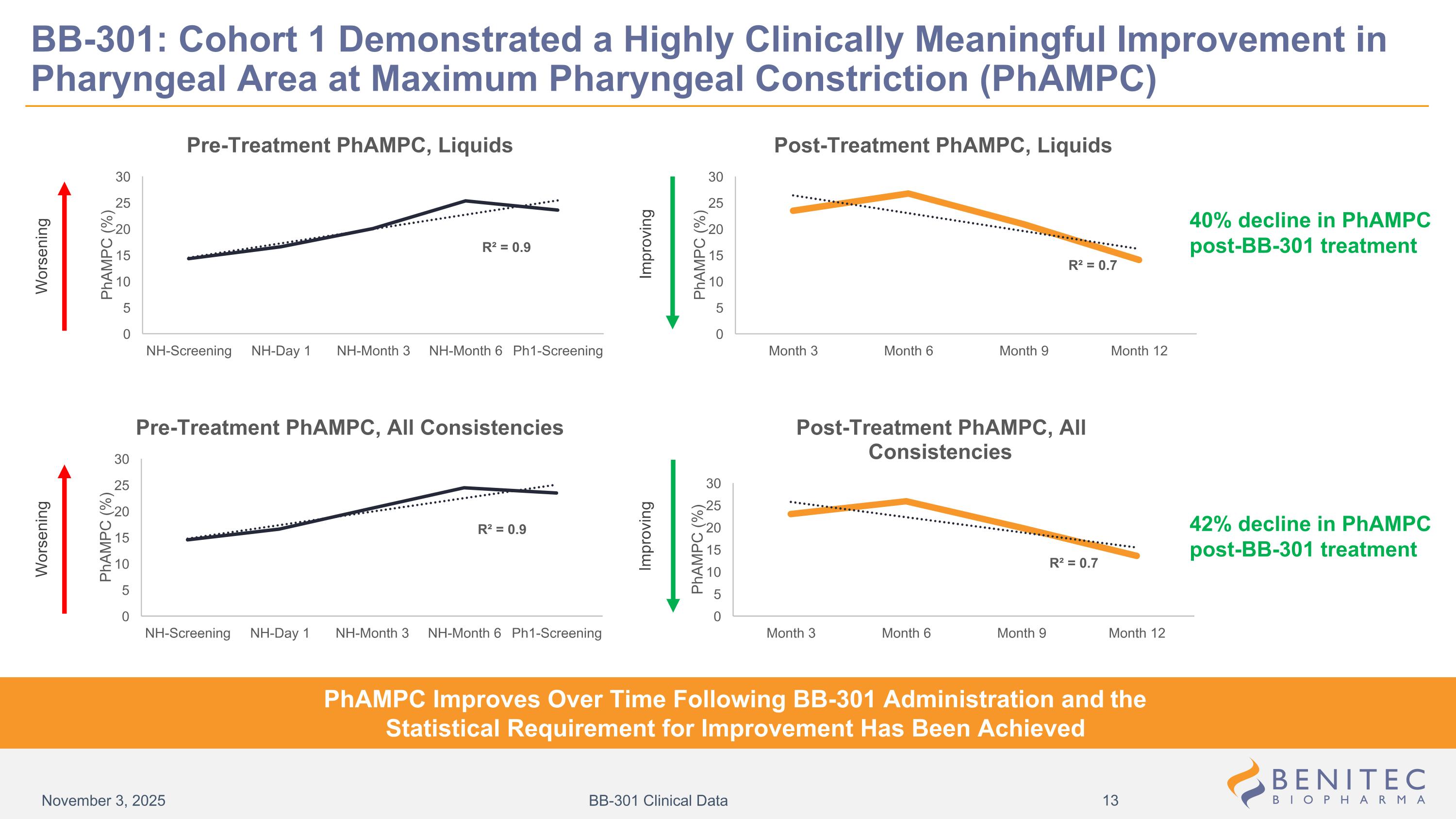
Worsening Improving Improving Worsening 40% decline in PhAMPC post-BB-301 treatment 42% decline in PhAMPC post-BB-301 treatment BB-301: Cohort 1 Demonstrated a Highly Clinically Meaningful Improvement in Pharyngeal Area at Maximum Pharyngeal Constriction (PhAMPC) PhAMPC Improves Over Time Following BB-301 Administration and the Statistical Requirement for Improvement Has Been Achieved November 3, 2025 BB-301 Clinical Data
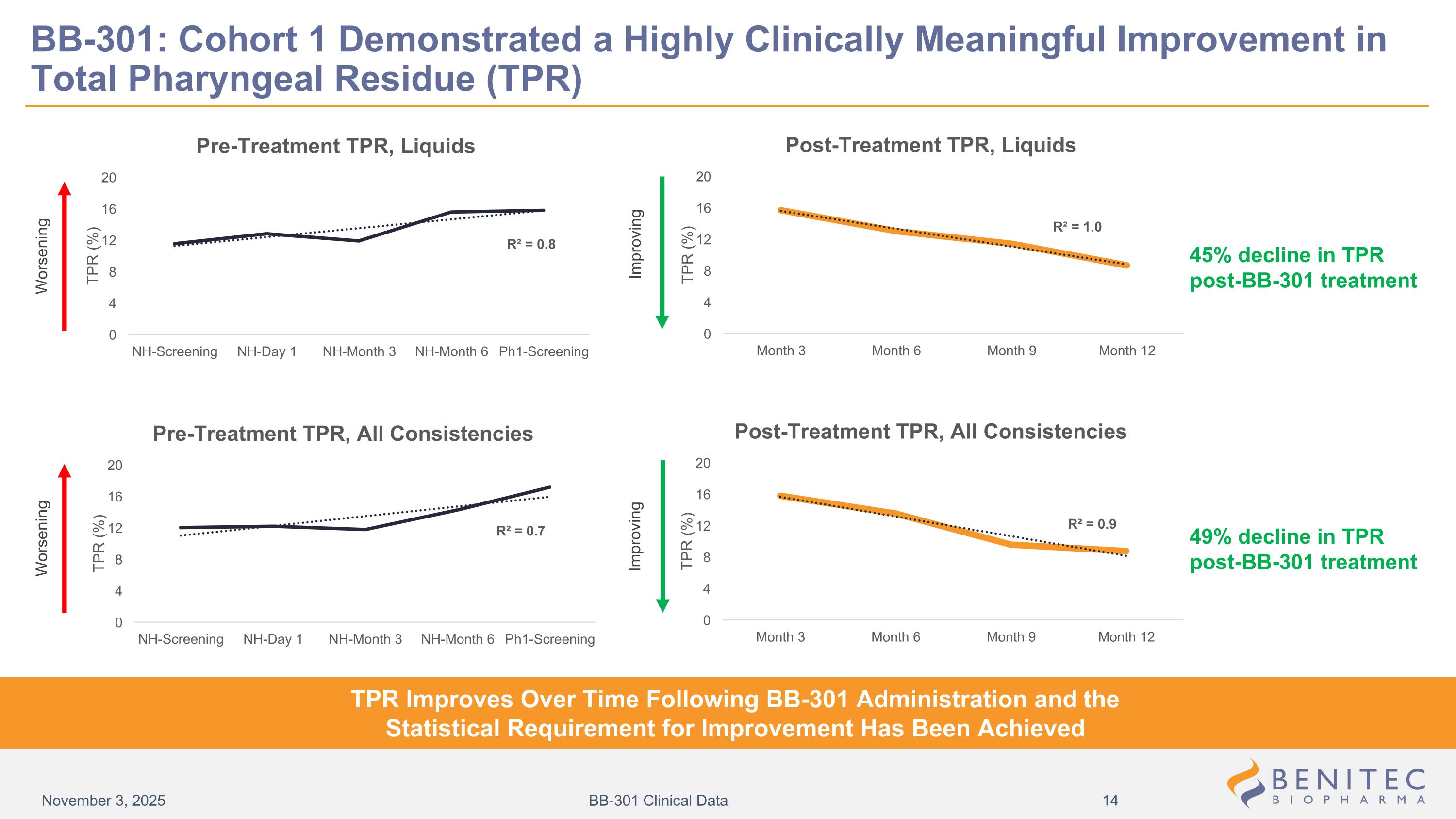
BB-301: Cohort 1 Demonstrated a Highly Clinically Meaningful Improvement in Total Pharyngeal Residue (TPR) Worsening Improving Improving Worsening TPR Improves Over Time Following BB-301 Administration and the Statistical Requirement for Improvement Has Been Achieved 45% decline in TPR post-BB-301 treatment 49% decline in TPR post-BB-301 treatment November 3, 2025 BB-301 Clinical Data
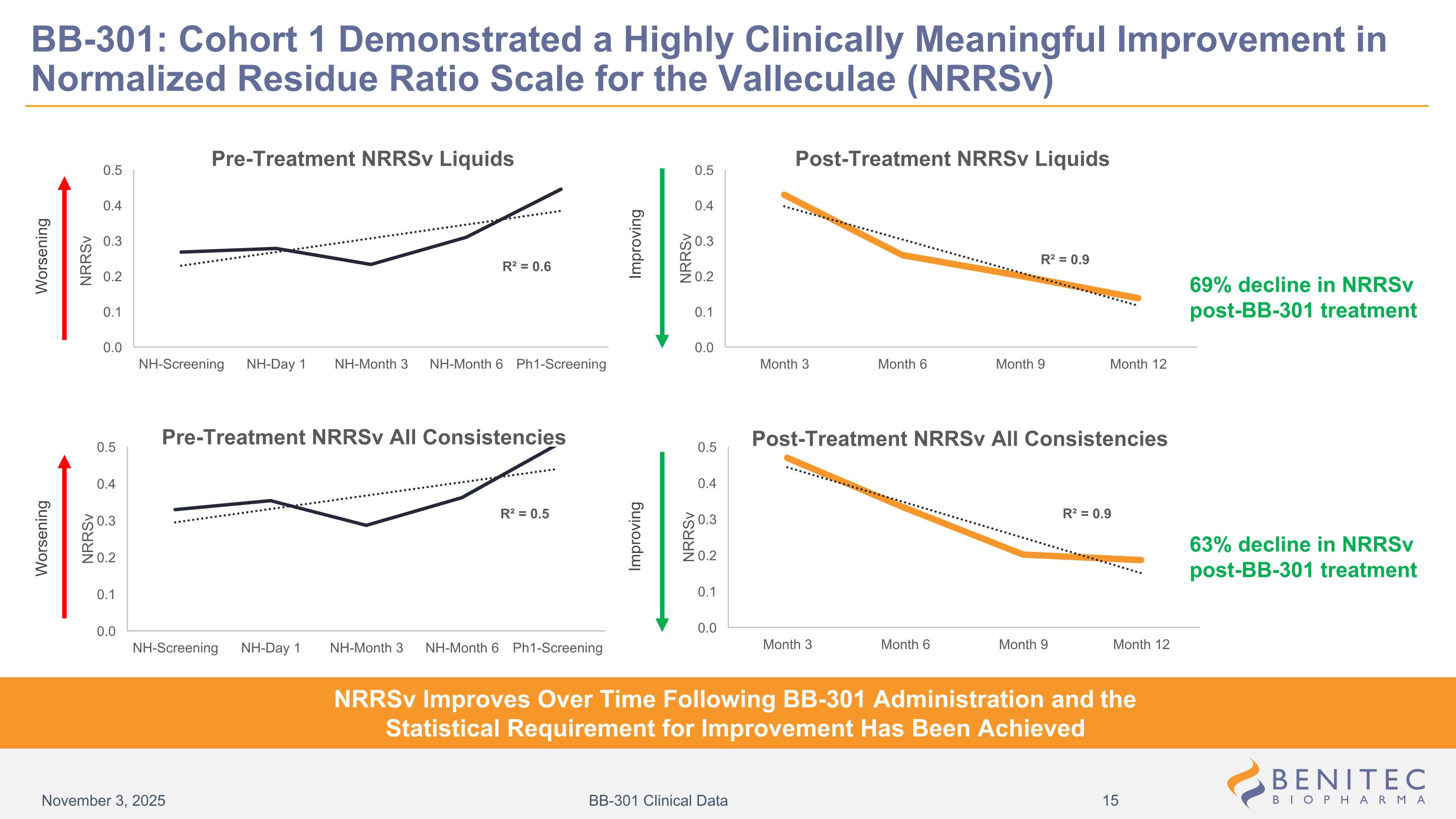
BB-301: Cohort 1 Demonstrated a Highly Clinically Meaningful Improvement in Normalized Residue Ratio Scale for the Valleculae (NRRSv) 69% decline in NRRSv post-BB-301 treatment 63% decline in NRRSv post-BB-301 treatment NRRSv Improves Over Time Following BB-301 Administration and the Statistical Requirement for Improvement Has Been Achieved Worsening Improving Improving Worsening November 3, 2025 BB-301 Clinical Data
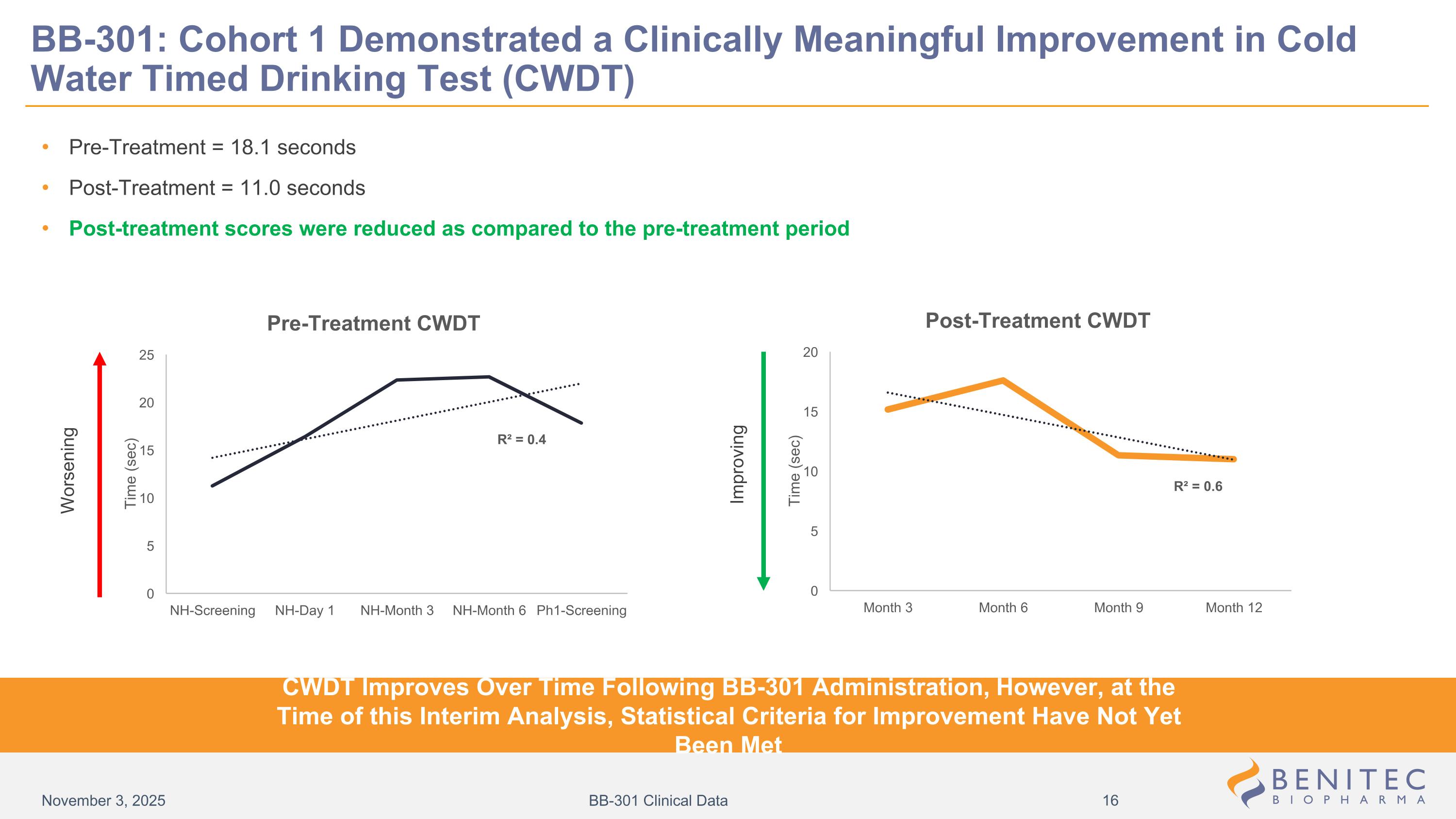
Pre-Treatment = 18.1 seconds Post-Treatment = 11.0 seconds Post-treatment scores were reduced as compared to the pre-treatment period Worsening Improving BB-301: Cohort 1 Demonstrated a Clinically Meaningful Improvement in Cold Water Timed Drinking Test (CWDT) CWDT Improves Over Time Following BB-301 Administration, However, at the Time of this Interim Analysis, Statistical Criteria for Improvement Have Not Yet Been Met November 3, 2025 BB-301 Clinical Data
Without intervention, the functional elements of the swallowing process worsen over time for OPMD patients, as shown in the pre-treatment results for Cohort 1 patients. The progressive loss of swallowing function drives the clinically significant symptom burden experienced by the OPMD patients. Pre-treatment, Cohort 1 patients exhibited worsening pharyngeal residue post-swallow (TPR and NRRSv), worsening pharyngeal area at maximum pharyngeal constriction (PhAMPC), abnormal total SSQ scores, and abnormal CWDT scores. Each patient enrolled into the BB-301 clinical development program serves as their own control. This approach was recently highlighted by the FDA in the Sept 2025 Draft Guidance for Industry entitled “Innovative Designs for Clinical Trials of Cellular and Gene Therapy Products in Small Populations” Evidence of stabilization or improvement, as seen post-BB-301 treatment for Cohort 1 patients, represents clinically meaningful improvement. Post BB-301 treatment, TPR, NRRSv, PhAMPC and SSQ results improved significantly. Post-treatment CWDT scores were reduced as compared to those observed in the pre-treatment period, however, at the time of this interim analysis the statistical criteria for the formal designation of improvement have not yet been met. BB-301: Natural History Trends of Cohort 1 Patients Inform Potential Pivotal Study Design and the Development of a Proprietary Responder Analysis November 3, 2025 BB-301 Clinical Data
BB-301 Responder Analyses: A Framework for Evaluation of Efficacy The total dysphagic symptom burden experienced by OPMD patients has several known underlying contributors Development of a multi-component composite endpoint to evaluate the potential treatment effects of BB-301 allows for incorporation of multiple discrete assessments that, in total, assess disease progression and treatment benefit of BB-301 The Responder Analysis is comprised of a combination of patient-reported outcome results, objective assessment results, and swallowing capacity assessment results: Patient-Reported Outcome Assessment results include: Sydney Swallow Questionnaire (SSQ) results Objective Assessment Results include: Videofluoroscopic swallowing study results (Pharyngeal Area at Maximum Pharyngeal Constriction [PhAMPC], Post-Swallow Pharyngeal Residue [Total Pharyngeal Residue or “TPR” and Normalized Residue Ratio Scale or “NRRS”], Frequency of sequential swallows “SEQ”) Functional Swallowing Capacity Assessment Results include: Clinically administered drinking assessment results (as measured by the cold-water timed drinking test “CWDT”) Results are combined into a single framework that facilitates the overall assessment of the clinical benefit achieved by each study patient. Interim clinical results using this analysis were submitted to the FDA over the course of an interactive process that supported the grant of Fast Track Designation for BB-301 November 3, 2025 BB-301 Clinical Data
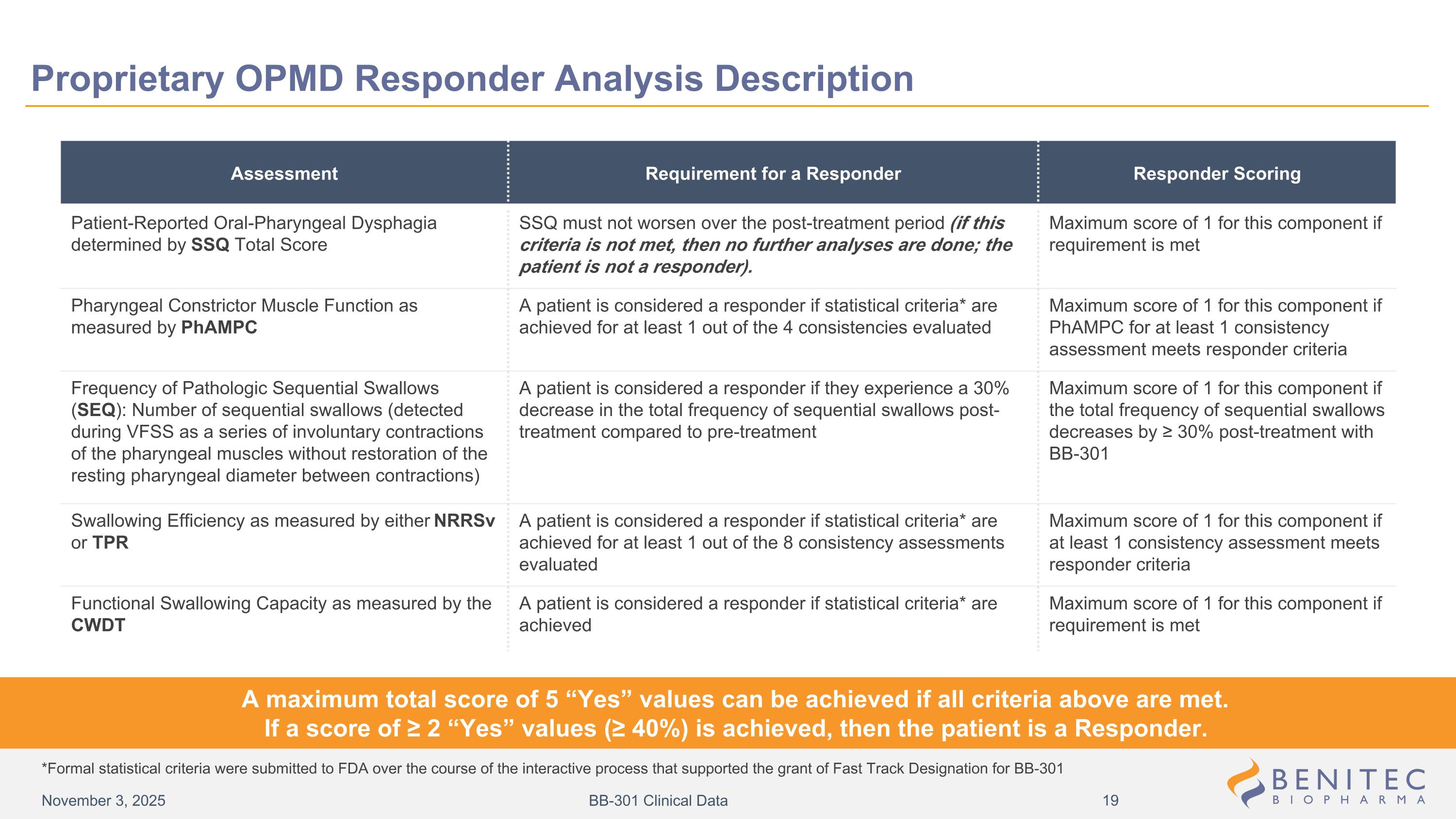
Assessment Requirement for a Responder Responder Scoring Patient-Reported Oral-Pharyngeal Dysphagia determined by SSQ Total Score SSQ must not worsen over the post-treatment period (if this criteria is not met, then no further analyses are done; the patient is not a responder). Maximum score of 1 for this component if requirement is met Pharyngeal Constrictor Muscle Function as measured by PhAMPC A patient is considered a responder if statistical criteria* are achieved for at least 1 out of the 4 consistencies evaluated Maximum score of 1 for this component if PhAMPC for at least 1 consistency assessment meets responder criteria Frequency of Pathologic Sequential Swallows (SEQ): Number of sequential swallows (detected during VFSS as a series of involuntary contractions of the pharyngeal muscles without restoration of the resting pharyngeal diameter between contractions) A patient is considered a responder if they experience a 30% decrease in the total frequency of sequential swallows post-treatment compared to pre-treatment Maximum score of 1 for this component if the total frequency of sequential swallows decreases by ≥ 30% post-treatment with BB-301 Swallowing Efficiency as measured by either NRRSv or TPR A patient is considered a responder if statistical criteria* are achieved for at least 1 out of the 8 consistency assessments evaluated Maximum score of 1 for this component if at least 1 consistency assessment meets responder criteria Functional Swallowing Capacity as measured by the CWDT A patient is considered a responder if statistical criteria* are achieved Maximum score of 1 for this component if requirement is met A maximum total score of 5 “Yes” values can be achieved if all criteria above are met. If a score of ≥ 2 “Yes” values (≥ 40%) is achieved, then the patient is a Responder. *Formal statistical criteria were submitted to FDA over the course of the interactive process that supported the grant of Fast Track Designation for BB-301 Proprietary OPMD Responder Analysis Description November 3, 2025 BB-301 Clinical Data
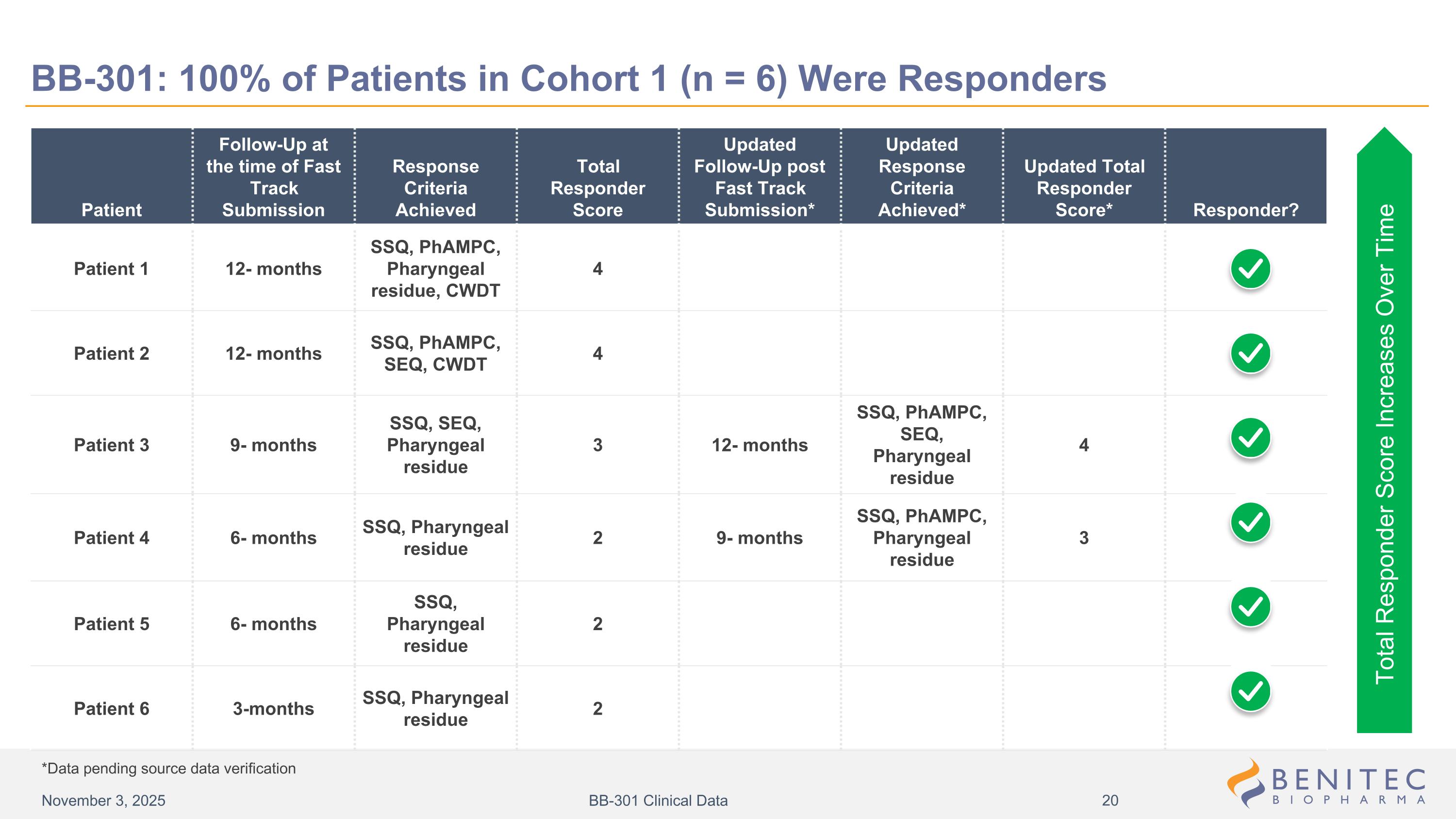
Patient Follow-Up at the time of Fast Track Submission Response Criteria Achieved Total Responder Score Updated Follow-Up post Fast Track Submission* Updated Response Criteria Achieved* Updated Total Responder Score* Responder? Patient 1 12- months SSQ, PhAMPC, Pharyngeal residue, CWDT 4 Patient 2 12- months SSQ, PhAMPC, SEQ, CWDT 4 Patient 3 9- months SSQ, SEQ, Pharyngeal residue 3 12- months SSQ, PhAMPC, SEQ, Pharyngeal residue 4 Patient 4 6- months SSQ, Pharyngeal residue 2 9- months SSQ, PhAMPC, Pharyngeal residue 3 Patient 5 6- months SSQ, Pharyngeal residue 2 Patient 6 3-months SSQ, Pharyngeal residue 2 Total Responder Score Increases Over Time *Data pending source data verification BB-301: 100% of Patients in Cohort 1 (n = 6) Were Responders November 3, 2025 BB-301 Clinical Data
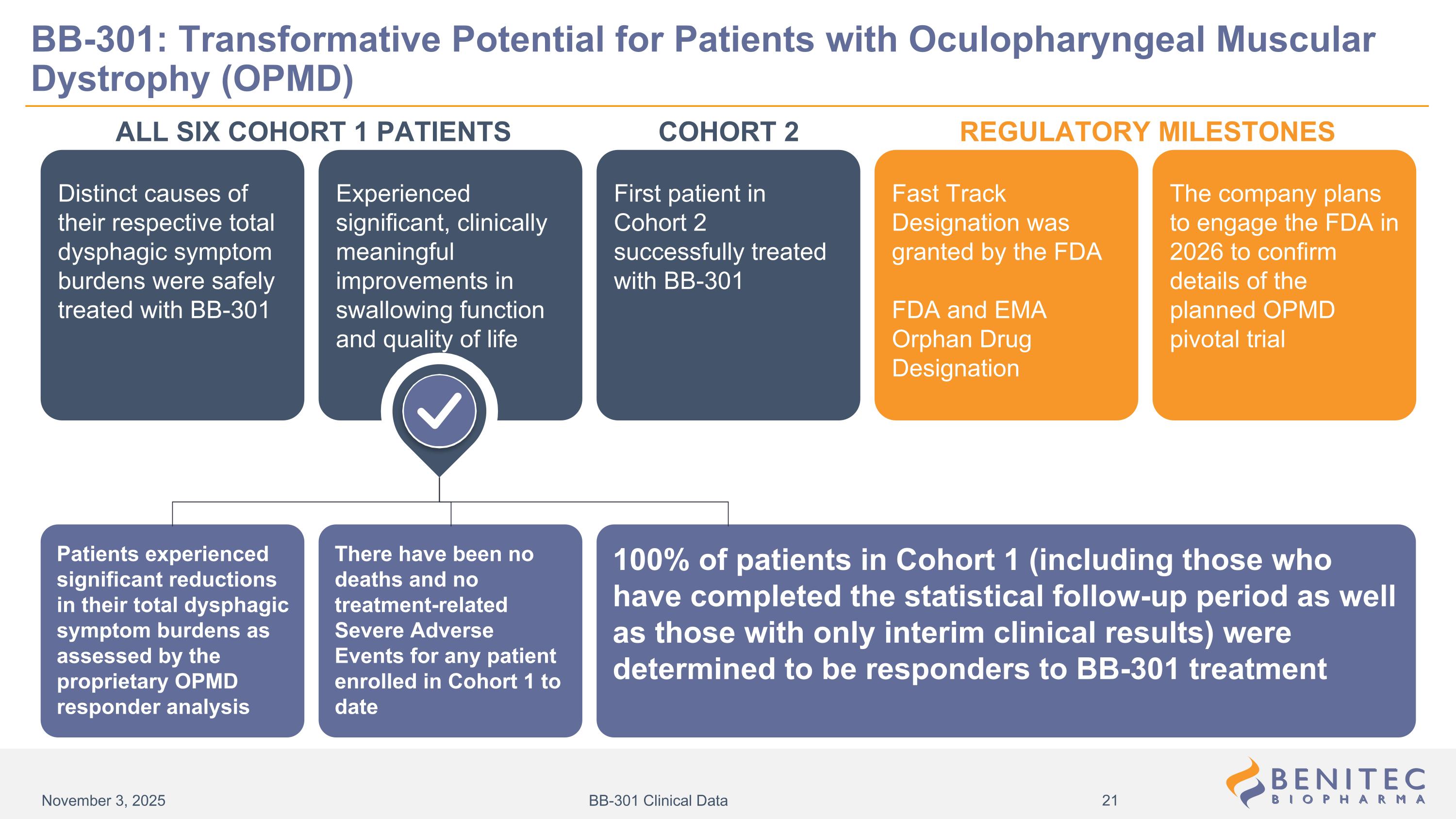
Distinct causes of their respective total dysphagic symptom burdens were safely treated with BB-301 Fast Track Designation was granted by the FDA FDA and EMA Orphan Drug Designation First patient in Cohort 2 successfully treated with BB-301 The company plans to engage the FDA in 2026 to confirm details of the planned OPMD pivotal trial Experienced significant, clinically meaningful improvements in swallowing function and quality of life ALL SIX COHORT 1 PATIENTS REGULATORY MILESTONES Patients experienced significant reductions in their total dysphagic symptom burdens as assessed by the proprietary OPMD responder analysis There have been no deaths and no treatment-related Severe Adverse Events for any patient enrolled in Cohort 1 to date 100% of patients in Cohort 1 (including those who have completed the statistical follow-up period as well as those with only interim clinical results) were determined to be responders to BB-301 treatment BB-301: Transformative Potential for Patients with Oculopharyngeal Muscular Dystrophy (OPMD) COHORT 2 November 3, 2025 BB-301 Clinical Data

www.benitec.com ©2025 Benitec Biopharma. All rights reserved. THANK YOU! NASDAQ BNTC

Appendix

OPMD-Related Dysphagia Natural History Study Data Informs Putative Pivotal Study Design and a Potential Proprietary Responder Analysis Transformative BB-301 Phase 1b/2a Clinical Study Results High Unmet Need and Significant Market Opportunity There are no therapeutic interventions approved for OPMD BB-301 is the only clinical-stage therapeutic agent in development designed to treat dysphagia (loss of the ability to swallow) in patients with OPMD 15,000 patients in the U.S., Europe, Canada, and Israel BB-301 is delivered via direct intramuscular (IM) injection to pharyngeal constrictor muscles resulting in minimal systemic exposure; 6 patients have been safely treated with the low dose of BB-301 and there have been no treatment related severe adverse events Following the administration of BB-301, Cohort 1 patients experienced significant reductions in dysphagic symptom burden, reductions in post-swallow residue accumulation, reductions in the time required to consume fixed volumes of liquid, and improvements in pharyngeal closure during swallowing All 6 patients enrolled into Cohort 1 met the formal statistical criteria for Response to BB-301, representing a 100% response rate Progressive dysphagia impacts 97% of OPMD patients and is a severe, life-threatening complication of OPMD which can lead to chronic choking, malnutrition, aspiration pneumonia, and death Without intervention, the functional elements of the swallowing process worsened over time for OPMD patients prior to their enrollment into Cohort 1 of the BB-301 Phase 1b/2a Clinical Treatment Study The Natural History Study results facilitated the development of a putative proprietary Responder Analysis and potential pivotal study design, both of which were presented to the FDA in support of Fast Track Designation for BB-301 BB-301: Potential One-Time Treatment Designed to Address the Root Cause of Oculopharyngeal Muscular Dystrophy (OPMD)-Related Dysphagia Benitec continues to plan for a formal meeting with FDA in 2026 to confirm all key elements of the anticipated BB-301 Pivotal Study design November 3, 2025 BB-301 Clinical Data
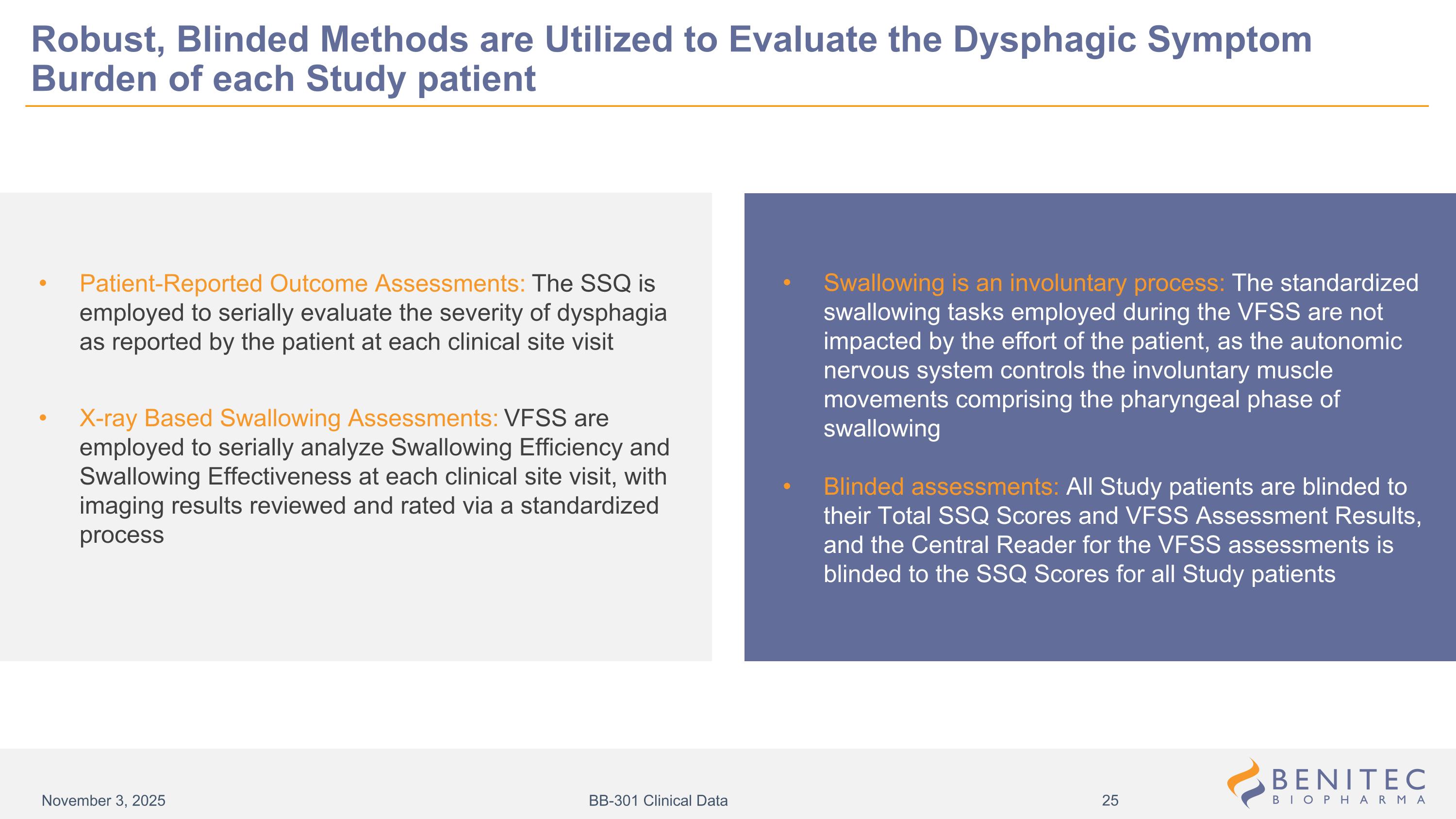
Swallowing is an involuntary process: The standardized swallowing tasks employed during the VFSS are not impacted by the effort of the patient, as the autonomic nervous system controls the involuntary muscle movements comprising the pharyngeal phase of swallowing Blinded assessments: All Study patients are blinded to their Total SSQ Scores and VFSS Assessment Results, and the Central Reader for the VFSS assessments is blinded to the SSQ Scores for all Study patients Patient-Reported Outcome Assessments: The SSQ is employed to serially evaluate the severity of dysphagia as reported by the patient at each clinical site visit X-ray Based Swallowing Assessments: VFSS are employed to serially analyze Swallowing Efficiency and Swallowing Effectiveness at each clinical site visit, with imaging results reviewed and rated via a standardized process Robust, Blinded Methods are Utilized to Evaluate the Dysphagic Symptom Burden of each Study patient November 3, 2025 BB-301 Clinical Data
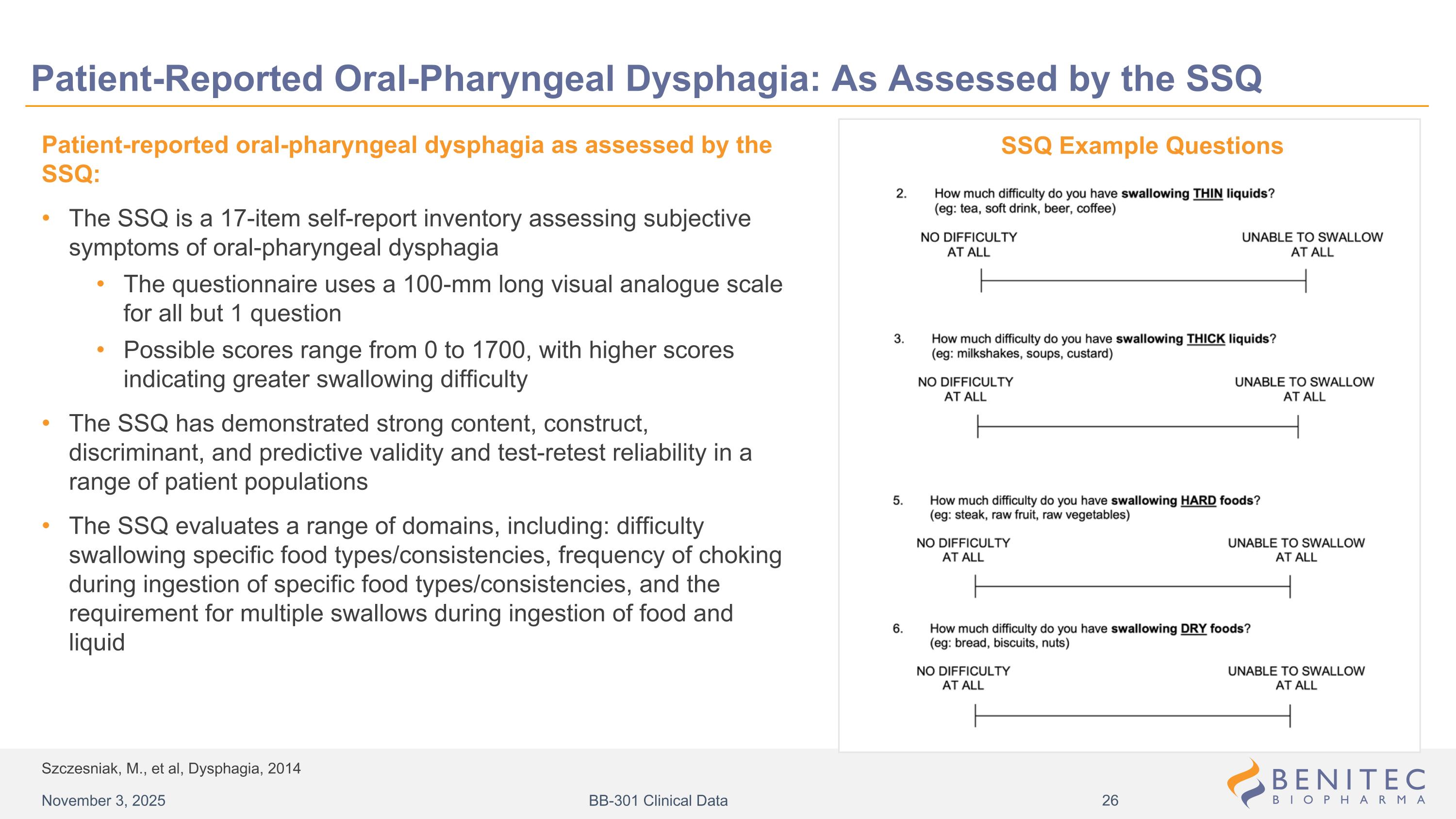
Szczesniak, M., et al, Dysphagia, 2014 Patient-Reported Oral-Pharyngeal Dysphagia: As Assessed by the SSQ Patient-reported oral-pharyngeal dysphagia as assessed by the SSQ: The SSQ is a 17-item self-report inventory assessing subjective symptoms of oral-pharyngeal dysphagia The questionnaire uses a 100-mm long visual analogue scale for all but 1 question Possible scores range from 0 to 1700, with higher scores indicating greater swallowing difficulty The SSQ has demonstrated strong content, construct, discriminant, and predictive validity and test-retest reliability in a range of patient populations The SSQ evaluates a range of domains, including: difficulty swallowing specific food types/consistencies, frequency of choking during ingestion of specific food types/consistencies, and the requirement for multiple swallows during ingestion of food and liquid SSQ Example Questions November 3, 2025 BB-301 Clinical Data
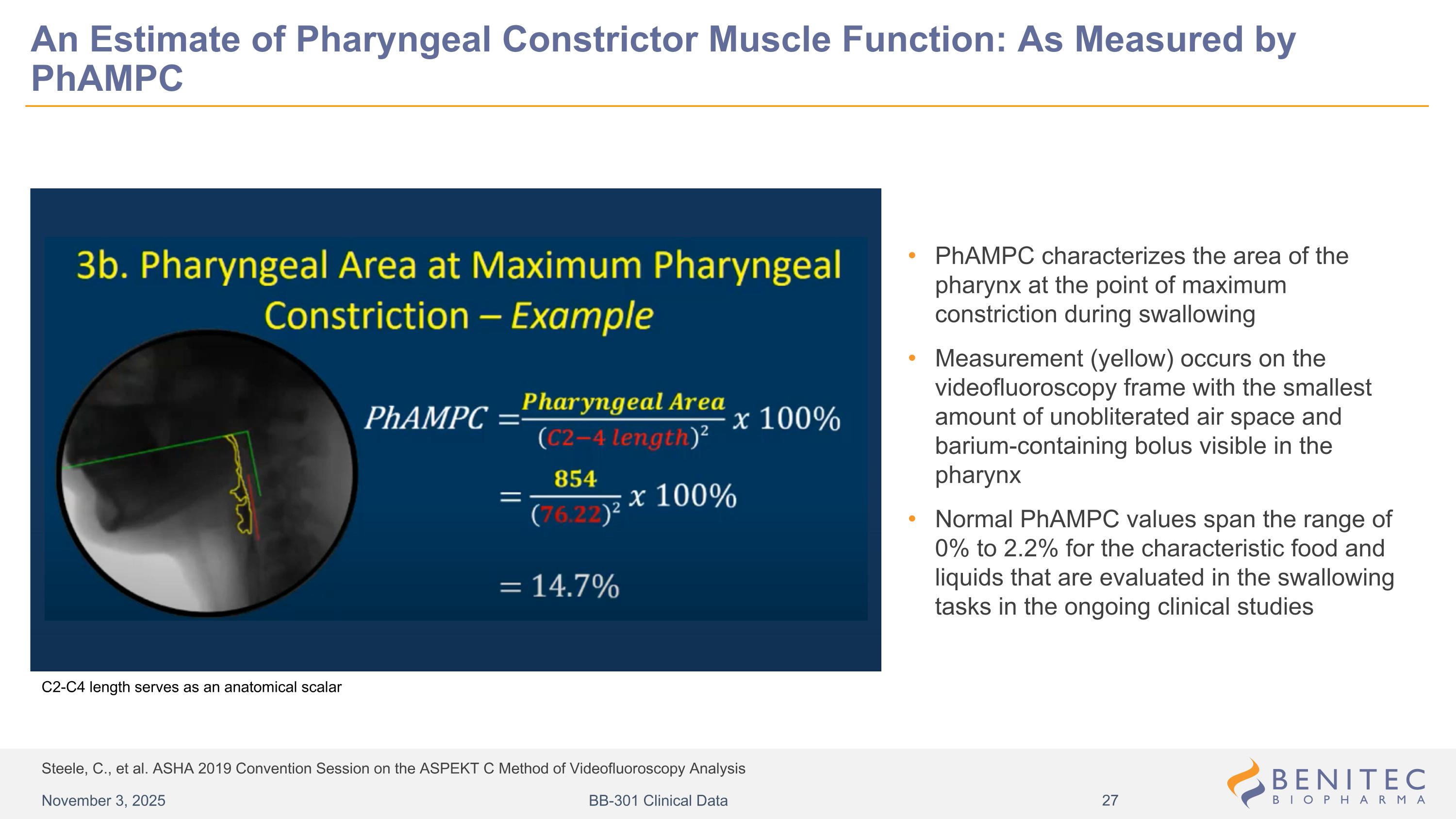
Steele, C., et al. ASHA 2019 Convention Session on the ASPEKT C Method of Videofluoroscopy Analysis An Estimate of Pharyngeal Constrictor Muscle Function: As Measured by PhAMPC PhAMPC characterizes the area of the pharynx at the point of maximum constriction during swallowing Measurement (yellow) occurs on the videofluoroscopy frame with the smallest amount of unobliterated air space and barium-containing bolus visible in the pharynx Normal PhAMPC values span the range of 0% to 2.2% for the characteristic food and liquids that are evaluated in the swallowing tasks in the ongoing clinical studies C2-C4 length serves as an anatomical scalar November 3, 2025 BB-301 Clinical Data
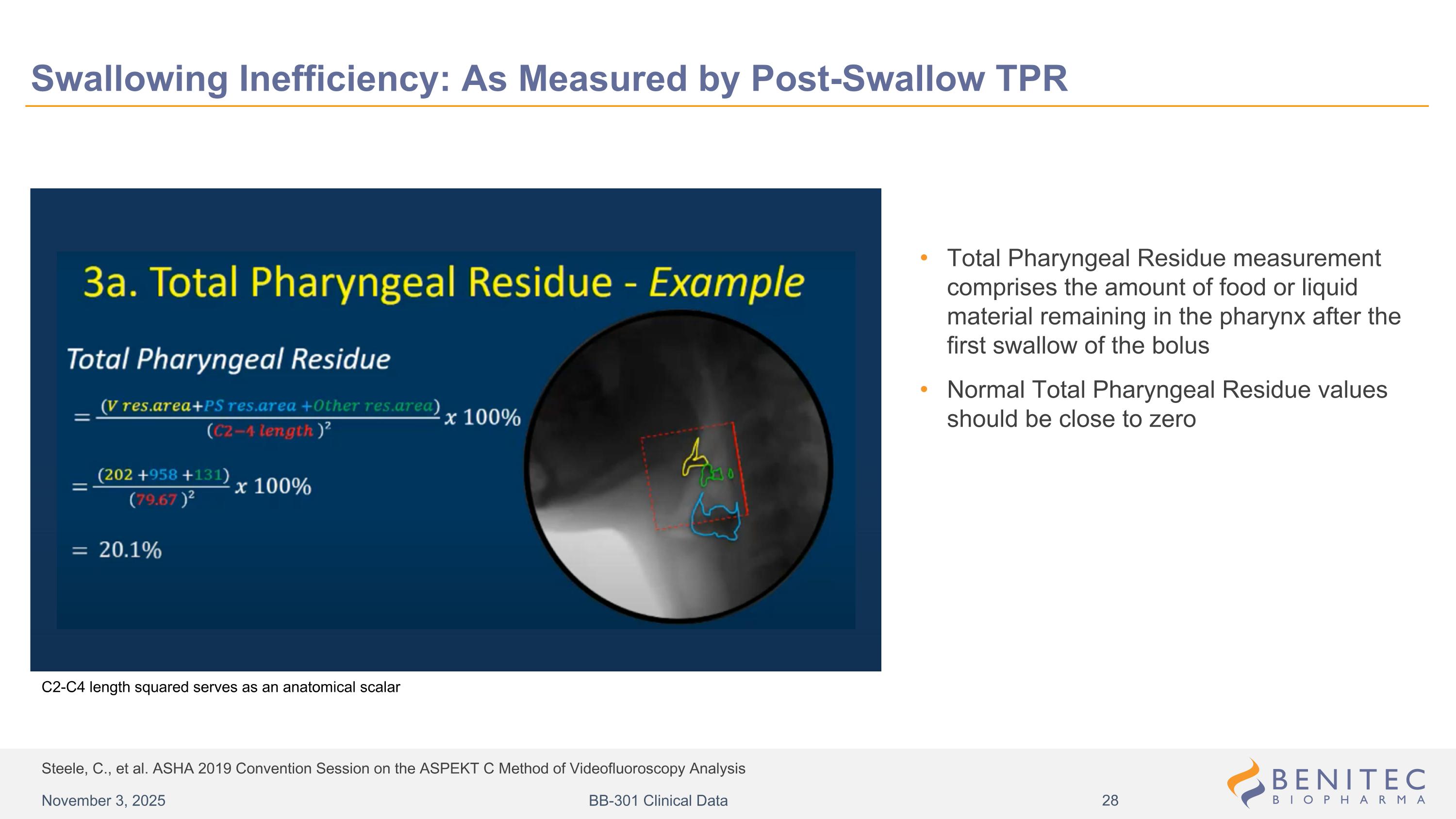
Steele, C., et al. ASHA 2019 Convention Session on the ASPEKT C Method of Videofluoroscopy Analysis Swallowing Inefficiency: As Measured by Post-Swallow TPR Total Pharyngeal Residue measurement comprises the amount of food or liquid material remaining in the pharynx after the first swallow of the bolus Normal Total Pharyngeal Residue values should be close to zero C2-C4 length squared serves as an anatomical scalar November 3, 2025 BB-301 Clinical Data

1. Pearson W.G. et. al., Dysphagia, 2013; 2. Steele, C.M. et. al., Journal of Speech, Language and Hearing Research, 2019; 3. Molfenter S.M. et. al., Dysphagia, 2013; 4. Kroon, R.H.M.J.M. et. al., Neurology, 2021 Swallowing Inefficiency: As Measured by NRRSv, a Measure of Vallecular Residue NRRS incorporates the ratio of residue occupying a pharyngeal space and a proportion expressing the area of observed residue normalized against the size of an individual1 Pharyngeal residue constitutes a risk for post-swallow aspiration, with clinically significant residue determined by NRRSv values ≥ 0.063 Residue accumulation in the valleculae (NRRSv ≥ 0.09) has also shown to be correlated with an increased risk of penetration-aspiration In a 2021 Dutch OPMD Natural History Study of 43-patients, a statistically significant decline in swallowing efficiency was captured via the use of longitudinal NRRSv assessments (2 assessments, occurring 20-months apart). The amount of abnormal pharyngeal residue in the valleculae increased significantly (mean NRRSv ratio 0.24 vs 0.13, p = 0.007) over this time period.4 Valleculae are the bilateral spaces between the base of the tongue and the epiglottis C2-C4 length squared (dashed red line) serves as an anatomical scalar and spatial housing areas are shown in white2 Measurement of residue (yellow) occurs on the first frame showing pyriform sinuses at lowest position2 November 3, 2025 BB-301 Clinical Data
1. Ambrocio, K. R., et al., Dysphagia (2023) 38:1497–1510; 2. Steele, C., et al., Journal of Speech, Language, and Hearing Research, Vol. 62, 1338–1363, May 2019 Swallowing Ineffectiveness: As Measured by the Frequency of Pathologic Sequential Swallows (SEQ) Sequential swallowing is defined as the completion of two or more consecutive swallows in rapid succession where the hyolaryngeal complex (HLC) does not return to rest and the pharynx lacks complete patency between swallows1 Under normal physiologic conditions, sequential swallowing occurs during continuous drinking of large volumes (90 mL or greater) of thin liquids via straw, cup, or bottle (for reference, one can of a soft drink has a volume of approximately 355 mL of thin liquid) For the BB-301 Phase 1b/2a Study, the return of the HLC to its resting position (i.e., achievement of pharyngeal muscle relaxation) between swallows is characterized on VFSS via the identification of the the “Swallow Rest Frame” Swallow Rest is the terminal event of each discrete swallow, and the Swallow Rest Frame is identified as the first VFSS frame showing the pyriform sinuses at their lowest position, relative to the spine, prior to any hyoid burst or laryngeal elevation for a subsequent subswallow2 In dysphagic disorders (e.g., OPMD), during the consumption of small volumes of liquids (e.g., less than 15 mL, as employed in the BB-301 Phase 1b/2a Study), pharyngeal muscle relaxation may not be achieved consistently between swallows (i.e., the HLC does not return to rest and the pharynx lacks complete patency); instead, a patient may experience pathologic sequential swallows when consuming small volumes of liquids Pathologic sequential swallows occur during the consumption of small volumes of liquid and are detected during VFSS as a series of involuntary contractions of the pharyngeal muscles without restoration of the resting pharyngeal diameter between contractions November 3, 2025 BB-301 Clinical Data

Functional Swallowing Capacity: As Measured by the CWDT 1. Nathardwarawala, K.M. et. al. Journal of Neurology, Neurosurgery, and Psychiatry, 1992; 2. Codère, F. et. al., Orbit, 2001; 3. Côté, C. et. al., Dysphagia, 2024; 4. Périé, S. et. al., Molecular Therapy, 2013 The CWDT measures the time required for a patient to swallow 80 mL of ice-cold water1 The CWDT (dysphagia documented by the inability to swallow 80 mL of cold water in less than 8 seconds), alongside family history of the disease and the presence of at least one ptotic eyelid with a fissure of less than 8 mm, can be used to make a clinical diagnosis of OPMD2 Failure to drink 80 mL of water in less than 8 seconds suggests the presence of dysphagia in OPMD individuals (sensitivity = 87% and specificity = 77%)3 The timed swallowing of 80 mL of cold water represents a global functional evaluation of swallowing and has been used as one of the endpoints to assess the outcome of an autologous myoblast transplant Phase 1/2a clinical study in OPMD patients4 November 3, 2025 BB-301 Clinical Data