Benitec Biopharma Provides Positive Updates on the BB-301 Pilot Dosing Study in Large Animals
HAYWARD, Calif., Sept. 8, 2021 /PRNewswire/ -- Benitec Biopharma Inc. (NASDAQ: BNTC), a development-stage biotechnology company focused on the advancement of novel genetic medicines, today provided an update on the ongoing analyses of the large animal subjects treated with BB-301 in the Pilot Dosing Study. Interim data from the BB-301 Pilot Dosing Study were disclosed in February 2021 via press release and presentation and subsequently discussed with European regulators in the first half of 2021. The updated BB-301 analyses outlined today continue to demonstrate robust, dose-dependent target tissue transduction, dose-dependent transgene expression, and biologically significant knock-down of the target protein. These data provide continued support for the planned advancement of BB-301 into the Phase 1b/2a study in 2022.
BB-301 is a novel investigational gene therapy under development for the treatment of patients with Oculopharyngeal Muscular Dystrophy (OPMD). OPMD is a chronic, life-threatening genetic disorder affecting approximately 15,000 patients in the United States, Canada, Western Europe, and Israel. OPMD is caused by a mutation in the gene encoding poly(A) binding protein nuclear 1 (PABPN1). Patients with OPMD lose the ability to swallow liquids and solids, and the natural history of the disorder is characterized by chronic malnutrition, aspiration, and fatal episodes of aspiration pneumonia. Currently, no therapeutic agents are approved for the treatment of OPMD. Additionally, no surgical interventions capable of altering the long-term natural history of OPMD are available.
Benitec has previously disclosed key data-points related to the completed non-clinical studies and the planned non-clinical studies for BB-301 that were anticipated to support the filing of Clinical Trial Applications in Europe and in Canada and, similarly, to facilitate the filing of an Investigational New Drug Application in the United States. The BB-301 Pilot Dosing Study in large animals (Beagle dogs) and the GLP Toxicology and Biodistribution Study represent two of the core non-clinical studies. BB-301 is directly injected into the pharyngeal muscles known to underlie the morbidity and mortality characterizing the natural history of OPMD. Against this backdrop, the BB-301 Pilot Dosing Study in large animal subjects was conducted to demonstrate that direct intramuscular injection of BB-301 via the use of a proprietary dosing procedure could safely achieve the following goals:
- Biologically significant, dose-dependent levels of BB-301 tissue transduction (i.e., delivery of the multi-functional genetic construct into the target pharyngeal muscle cells)
- Broad-based, dose-dependent expression within the pharyngeal muscle cells of the three distinct genes comprising the BB-301 gene construct
- Biologically significant levels of target gene knock-down (i.e., inhibition of the expression of the gene of interest) within the pharyngeal muscle cells
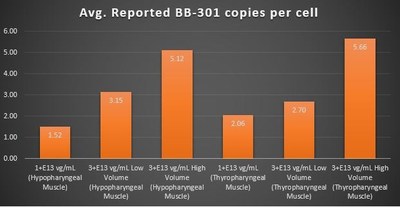
The BB-301 Pilot Dosing Study evaluated the safety and biological activity of two concentrations of BB-301 (1.0+E13 vg/mL and 3.0+E13 vg/mL) across three distinct doses (1.0+E13 vg/mL, 3.0+E13 vg/mL with a low injection volume, and 3.0+E13 vg/mL with a high injection volume) following direct intramuscular injection into the Hypopharyngeus (HP) muscles and the Thyropharyngeus (TP) muscles of Beagle dogs. The HP muscle in Beagle dogs corresponds to the Middle Pharyngeal Constrictor muscle in Human subjects, and the TP muscle in Beagle dogs corresponds to the Inferior Pharyngeal Constrictor muscle in Human subjects. BB-301 was injected only on Day 1 of the Pilot Dosing Study, and the corresponding canine pharyngeal muscles were harvested for analysis after 8 weeks of follow-up. BB-301 dosing was carried out by both a veterinary surgeon and a practicing otolaryngologist who has extensive experience with the provision of palliative surgical care for OPMD patients.
The initial interim data-points from the BB-301 Pilot Dosing Study presented in February 2021 via press release and presentation were derived from completed analyses of the pharyngeal muscle tissues isolated from 6 Beagle dog subjects. The updated analyses of the BB-301 Pilot Dosing Study subjects outlined today comprise data-points derived from 16 Beagle dog subjects. Further data analyses are ongoing for the canine subjects treated in the 24-subject BB-301 Pilot Dosing Study. The key updates are summarized below.
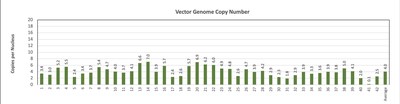
Pharyngeal Muscle Tissue Transduction for BB-301 (Figure 1):
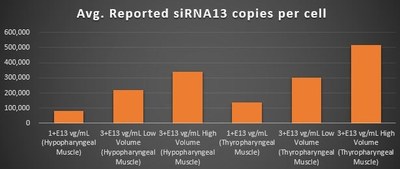
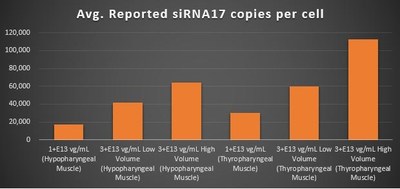
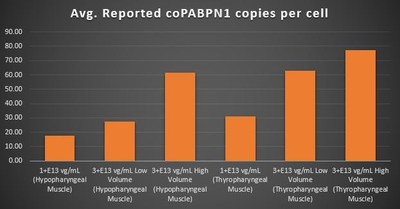
Gene Expression Within the Pharyngeal Muscle Tissues (Figure 2, Figure 3, Figure 4):
- BB-301 encodes two distinct siRNA species (i.e. siRNA13 and siRNA17) which are each, independently, capable of inhibiting (i.e. "silencing") the expression of the mutant form of the PABPN1 protein and the wild type (i.e. endogenous) form of the PABPN1 protein (importantly, the mutant form of the PABPN1 protein underlies the development and progression of OPMD)
- BB-301 also encodes a wild type version of the PABPN1 protein whose intracellular expression is unaffected by the inhibitory activities of siRNA13 and siRNA17, and this codon optimized PABPN1 protein (i.e. coPABPN1) serves to replenish the endogenous form of the PABPN1 protein and to replace the mutant form of PABPN1 that underlies the development and progression of OPMD in diseased tissues
- For comparative purposes, it should be noted that the average level of expression for wild type PABPN1 within the pharyngeal muscle cells of Beagle dogs is 4.5-to-7.8 copies per cell
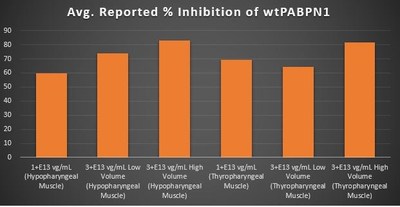
Wild Type PABPN1 Knock-Down Within the Pharyngeal Muscle Tissues (Figure 5):
- As noted above, BB-301 encodes two distinct siRNA species (i.e. siRNA13 and siRNA17) which are each, independently, capable of inhibiting (i.e. "silencing") the expression of all forms of the PABPN1 protein (siRNA13 and siRNA17 silence the expression of both wild type PABPN1 [wtPABPN1] and mutant PABPN1)
- While the Beagle dog subjects treated in the current BB-301 Pilot Dosing Study do not express mutant PABPN1, the level of BB-301-driven gene silencing for the PABPN1 target can be accurately assessed via the equivalent inhibitory effects of siRNA13 and siRNA17 on both wtPABPN1 and mutant PABPN1
- Thus, the wtPABPN1 silencing activity observed in the current BB-301 Pilot Dosing Study serves as a surrogate for the activity that would be anticipated in the presence of mutant PABPN1
- BB-301 has been evaluated in prior non-clinical studies in animals that express mutant PABPN1 and manifest the key signs and symptoms of OPMD and, in these animal models of OPMD, the achievement of PABPN1 silencing levels of 31% or higher led to complete resolution of OPMD disease symptoms and correction of the histological hallmarks of the disease
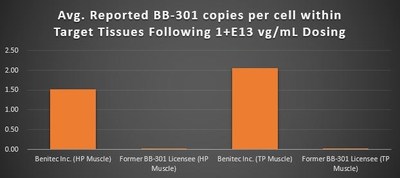
Ubiquity of Target Tissue Transduction Following Implementation of the Proprietary Dosing Procedure (Figure 6, Figure 7):
It is critical to highlight the key methodological distinctions between the current BB-301 Pilot Dosing Study conducted by Benitec as compared to the prior BB-301 Beagle dog dosing study carried out independently by the previous BB-301 licensee. The BB-301 dosing study conducted by the prior BB-301 licensee employed non-ideal routes and methods of BB-301 administration to the target pharyngeal muscle tissues and employed similarly limited analytical methods at the completion of the dosing phase of the study. The Benitec team worked to optimize the route and method of administration of BB-301 and to refine the core analytical methods employed following the completion of dosing.
The newly developed proprietary methods of BB-301 delivery, as well as the analytical methods developed to assay the key target tissues of the Beagle dog subjects, led to broad-based transduction of the pharyngeal muscle target tissues (Figure 6, individual sections of the TP muscle following BB-301 dosing) and demonstrated a 228-fold improvement (+22,647%) in BB-301 transduction of the HP muscle and a 113-fold improvement (+11,163%) in BB-301 transduction of the TP muscle relative to the levels of BB-301 transduction observed by the previous BB-301 licensee (Figure 7).
About Benitec Biopharma, Inc.
Benitec Biopharma, Inc. ("Benitec" or the "Company") is a development-stage biotechnology company focused on the advancement of novel genetic medicines with its headquarters in Hayward, California. The proprietary platform, called DNA-directed RNA interference, or ddRNAi, combines RNA interference, or RNAi, with gene therapy to create medicines that facilitate sustained silencing of disease-causing genes following a single administration. The Company is developing ddRNAi-based therapeutics for chronic and life-threatening human conditions including Oculopharyngeal Muscular Dystrophy (OPMD), and Chronic Hepatitis B. A comprehensive overview of the Company can be found on Benitec's website at www.benitec.com.
Forward Looking Statements
Except for the historical information set forth herein, the matters set forth in this press release represent forward-looking statements, including statements regarding BB-301, Benitec's plans to develop and commercialize its product candidates, the timing of the initiation and completion of preclinical and clinical trials, the timing of patient enrolment and dosing in clinical trials, the timing of expected regulatory filings, the clinical utility and potential attributes and benefits of ddRNAi and Benitec's product candidates, potential future out-licenses and collaborations, the intellectual property position and the ability to procure additional sources of financing, and other forward-looking statements. In addition, preliminary results or other preliminary analyses do not in any way ensure that later or final results in a clinical trial or in similar clinical trials will replicate those interim results.
These forward-looking statements are based on the Company's current expectations and subject to risks and uncertainties that may cause actual results to differ materially Some of the risks and uncertainties that may cause our actual results, performance or achievements to differ materially from those expressed or implied by forward-looking statements include the following:
- the success of our plans to develop and potentially commercialize our product candidates; the timing of the initiation and completion of preclinical studies and clinical trials;
- the timing and sufficiency of patient enrollment and dosing in any future clinical trials;
- the timing of the availability of data from clinical trials;
- the timing and outcome of regulatory filings and approvals;
- unanticipated delays;
- sales, marketing, manufacturing and distribution requirements;
- market competition and the acceptance of our products in the marketplace;
- regulatory developments in the United States;
- the development of novel AAV vectors;
- the plans of licensees of our technology;
- the clinical utility and potential attributes and benefits of ddRNAi and our product candidates;
- including the potential duration of treatment effects and the potential for a "one shot" cure;
- our dependence on our relationships with collaborators and other third parties;
- expenses, ongoing losses, future revenue, capital needs and needs for additional financing;
- the length of time over which we expect our cash and cash equivalents to be sufficient to execute on our business plan;
- our intellectual property position and the duration of our patent portfolio;
- the impact of local, regional, and national and international economic conditions and events; and
- the impact of the current COVID-19 pandemic, the disease caused by the SARS-CoV-2 virus, which may adversely impact our business and preclinical and future clinical trials;
as well as other risks detailed under the caption "Risk Factors" in our reports filed with the SEC from time to time. Any forward-looking statements in this release speak only as of the date on which it was made. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise.
Media & Investor Relations Contact:
Jay A. Morakis
CEO of M Group Strategic Communications (for Benitec Biopharma, Inc.)
Phone: 646-859-5951
Email: jmorakis@mgroupsc.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/benitec-biopharma-provides-positive-updates-on-the-bb-301-pilot-dosing-study-in-large-animals-301371241.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/benitec-biopharma-provides-positive-updates-on-the-bb-301-pilot-dosing-study-in-large-animals-301371241.html
SOURCE Benitec Biopharma Inc.
Released September 8, 2021